Bronchodilator Therapy: Introduction
Bronchodilators relax constricted airway smooth muscle in vitro. Because of this property, bronchodilators reverse airway obstruction, prevent bronchoconstriction and provide protection from constrictor stimuli.1 In this chapter, bronchodilators employed in mechanically ventilated patients are discussed with a special emphasis on inhalation therapy.
Rationale
Ventilator-dependent patients with acute exacerbations of chronic obstructive pulmonary disease (COPD) and acute severe asthma routinely receive bronchodilators to relieve bronchoconstriction. By reducing airway resistance, bronchodilators reduce the pressure required to ventilate the lung. This reduction in pressure may protect the lung against injury and enhance patient comfort. A general population of ventilated patients in a medical intensive care unit (ICU)2,3 and patients with acute respiratory distress syndrome4,5 showed improvement in expiratory airflow and airway resistance after bronchodilators. Infants with bronchopulmonary dysplasia, and children with asthma, or bronchiolitis also receive bronchodilators on a routine basis.6–10 In ventilated patients with COPD, elevated airway resistance and intrinsic positive end-expiratory pressure are major causes for weaning failure.11 In these patients, bronchodilators may facilitate weaning.12 Therapy with bronchodilators is, therefore, routinely and commonly employed for many indications in ventilator-dependent patients.13
Pharmacologic Agents
β-adrenergic agonists, anticholinergic drugs, and methylxanthines are the three major classes of bronchodilators employed in the ICU. β-adrenergic agonists and anticholinergics are usually administered by the inhaled route, whereas methylxanthines can only be administered enterally or parenterally. Although they are not bronchodilators in the classic sense, corticosteroids, both inhaled and systemic, are commonly employed in acutely ill patients to reduce airway inflammation and increase airway caliber.14–16
The pharmacology of the β-agonists was extensively reviewed in the second edition of this textbook,17 and other excellent reviews are available.18
β-agonists have been given by oral, subcutaneous, intravenous, and inhaled routes. Table 63-1 lists doses for individual drugs. Inhaled therapy is preferred because the drug is delivered directly to its site of action in the airways, a smaller quantity of drug produces an effect comparable to that observed with systemic administration, onset of effect is rapid, and systemic absorption of the drug is limited, thus minimizing side effects. The oral approach has been all but abandoned, and there appears to be no advantage to the intravenous route even in severe asthma with hypercapnia.19 A meta-analysis found no evidence of benefit for the intravenous use of β-agonists in patients who are refractory to inhaled β-agonists.20
| Agents | Dose | Time Course (Onset, Peak, Duration) | Frequency of Dosing |
|---|---|---|---|
| Albuterol (Salbutamol) | SVN: 0.083% solution, 3 mL (2.5 mg), pMDI: 90 mcg/puff, 4 puffs | 5 to 15 minutes, 30 to 60 minutes, 5 to 8 hours | 4 to 6 times daily |
| Levalbuterol | SVN: 0.63 mg, or 1.25 mg | 15 minutes, 30 to 60 minutes, 5 to 8 hours | 3 to 4 times daily |
| Formoterol | SVN: 20 mcg/2 mL solution | 1 to 3 minutes, 1 to 3 hours, 8 to 12 hours | 2 times daily |
| Arformoterol | SVN: 15 mcg/2ml solution | 1 to 3 minutes, 1 to 3 hours, 8 to 12 hours | 2 times daily |
| Ipratropium | pMDI: 18 mcg/puff, 4 puffs SVN: 0.02% solution, 2.5 mL (0.5 mg) | 15 minutes, 90 to 120 minutes, 6 to 8 hours | 4 to 6 times daily |
| Albuterol + Ipratropium | pMDI: 90 mcg + 18 mcg/puff, 4 puffs SVN: 2.5 mg + 0.5 mg/dose | 5 to 15 minutes, 30 to 60 minutes, 6 to 8 hours | 4 to 6 times daily |
Albuterol is today’s standard short-acting bronchodilator. Its pharmacokinetics depend on the dose administered, the formulation of albuterol used (dry powder, pressurized metered-dose inhaler [pMDI], or nebulized) and the clinical situation (mechanically ventilated or ambulatory). Although increasing doses of albuterol produce greater bronchodilation, the optimum dose is difficult to predict. Peak bronchodilator response after 10 puffs of albuterol in ventilated patients was seen within 5 minutes and sustained for 60 minutes.12 Duration of action of 6 puffs of albuterol delivered from a pMDI to ventilated patients ranged from less than 2 hours to more than 4 hours.21 Systemic effects are dose related, usually appearing within an hour of administration with a return to baseline within 4 hours.22
Commonly available racemic albuterol is a 50-50 mixture of R-albuterol and S-albuterol. Levalbuterol, the R-enantiomer, was formulated to avoid possible adverse effects of the S-enantiomer. For doses that produce similar increases in forced expiratory volume in 1 second (FEV1), increases in heart rate are less with levalbuterol (2 to 4 beats/min) than with albuterol,23 and the bronchodilator effect is greater in patients with acute asthma.24,25 In patients receiving long-term mechanical ventilation, levalbuterol produced an increase in secretion volume but the effect dissipated rapidly.26
Salmeterol is a long-acting β2-agonist. Its onset of action is slower than that of albuterol in vitro (mean: 6.4 vs. 1.9 minutes)27 and in vivo (mean: 10 vs. 4 minutes).28 With salmeterol, peak bronchodilator response was seen at 5 hours versus 1 hour with albuterol, and FEV1 was higher than predose FEV1 levels for 12 hours.28 Systemic effects of salmeterol are dose related, manifest later, and last longer; maximum effects on heart rate occur within 75 to 135 minutes and are still evident after 4 hours.22 In ventilated patients with acute exacerbations of COPD, four doses of salmeterol by pMDI (100 mcg) produced a bronchodilator effect within 30 minutes that was maintained for approximately 8 hours, with significant variability in the magnitude and duration of the response among patients.29
Formoterol is a long-acting agent like salmeterol but is distinguished by its relatively quicker onset of action.30 Duration of effect is approximately 12 hours. Because of its rapid onset of effect, formoterol is effective during acute exacerbations.31 Stereoselective formoterol (arformoterol) is available as a nebulized solution.
Indacaterol, vilanterol, and carmoterol have duration of action of 24 hours or more and are in various stages of clinical development.32
The principal mechanism of action of muscarinic antagonists is to block vagally mediated bronchoconstriction. In patients with COPD, a greater bronchodilator response occurs after atropine than after albuterol. The bronchodilator response to atropine in patients with COPD is greater than that in normal subjects. These data suggest enhanced parasympathetic activity in COPD.33,34 Patients with chronic asthma do not show as much benefit with anticholinergic agents, although patients with acute asthma exacerbations benefit from inhaled anticholinergic agents.
The quaternary ammonium-containing muscarinic antagonists in current use have no significant effects on mucus secretion or the rheologic properties of mucus,35 although some reports do suggest a decrease in sputum volume.36 Mucociliary clearance is unchanged with inhaled ipratropium.37,38
Acetylcholine can stimulate alveolar macrophages to release chemotactic factors.39 Long-acting anticholinergics may thus decrease airway inflammation by blocking the actions of acetylcholine.40
Although muscarinic receptors are widespread in the body, the quaternary ammonium group in modern antimuscarinic agents (ipratropium and tiotropium) results in poor systemic absorption allowing higher doses to be inhaled with few systemic effects.
Dryness of the mouth is a common side effect of anticholinergics, whereas blurring of vision occurs infrequently. Unilateral mydriasis is believed to result from direct contact of the nebulized solution with the eyes.41,42 Ipratropium inhalation does not worsen arterial oxygenation,43 and tolerance to the bronchodilator or bronchoprotective effects of anticholinergics does not occur. Systemic side effects (tachycardia, palpitations, urinary hesitancy, constipation, blurred vision, or glaucoma) are more common with tertiary ammonium compounds, such as atropine, than with ipratropium or tiotropium. In the Lung Health Study, Anthonisen et al found a higher incidence of supraventricular arrhythmias as well as significant increases in hospitalization and mortality rates in the ipratropium group as compared to the placebo group.44 A slightly higher risk of developing cerebrovascular accidents was suggested for patients receiving tiotropium (), and a metaanalysis found a higher risk of cardiovascular events in patients with COPD receiving ipratropium or tiotropium,45 although these have not been observed in a recently concluded large randomized controlled trial.46 Nevertheless, ipratropium and tiotropium should be used with caution in patients with renal failure, prostatic hypertrophy, urinary retention, or glaucoma.
The usual dose is 36 mcg (2 puffs from a pMDI) every 4 to 6 hours. There is a plateau in the achievable improvement in FEV1 with increasing doses with no significant improvement beyond a dose of 72 mcg.47 In patients with severe airway obstruction, however, the amount of drug delivered to the site of action may vary and higher doses may be needed. The usual nebulized dose is 500 mcg every 6 hours. The onset of action is within 30 minutes of an inhaled dose and the effects last for about 4 to 6 hours.
Tiotropium is a long-acting bronchodilator that has equal binding affinity for M1, M2, and M3 receptor subtypes. It dissociates much more slowly from M3 receptors than from M2 receptors.48 In view of the role of M2 receptors in inhibiting acetylcholine release, this may represent a beneficial aspect of its action. Tiotropium is inhaled once daily. Onset of action is within 30 minutes. An increase in FEV1 from baseline is present at 24 hours.49
Corticosteroids have no direct action on contraction of airway smooth muscle; hence, they are not bronchodilators in the classic sense. Corticosteroids, however, offer bronchoprotection, and they improve airway obstruction by reducing airway inflammation and via vasoconstrictor effects.16,50 Corticosteroids reduce airway inflammation by several mechanisms,51–54 decrease airway hyperresponsiveness and reduce the predilection to acute episodes of airflow obstruction. Corticosteroids also upregulate β-receptor expression on cell membranes, increase the proportion of β receptors in a high-affinity binding state, and inhibit the release of inflammatory mediators such as phospholipase A2, which could destabilize membrane support of the β receptor.55
Several corticosteroids, such as hydrocortisone, cortisone, prednisone, prednisolone, and methylprednisolone are given parenterally or orally for treatment of inflammation in asthma and COPD. Agents with high topical activity are employed as inhaled corticosteroids (ICSs), such as beclomethasone, triamcinolone, flunisolide, fluticasone, budesonide, mometasone, and ciclesonide, because they produce direct antiinflammatory effects while minimizing unwanted systemic side effects.
Systemic administration of corticosteroids is associated with several adverse side effects.56 The use of ICSs is attractive mainly to reduce the side effects observed with systemic therapy.57,58 Side effects of therapy with ICSs include oropharyngeal fungal infections, hoarseness, hypothalamic–pituitary–adrenal axis suppression, reduction in bone mass, growth restriction in children, skin bruising, and increased propensity to develop pneumonia.57–60
Patients presenting with acute severe asthma and acute exacerbations of COPD are in urgent need of bronchodilation. Inhalation is the preferred route of administration of bronchodilator agents because it produces a rapid onset of action, requires smaller doses than those needed orally or parenterally, and minimizes systemic side effects.61 For administration of short-acting β-agonists the use of pMDI and holding chamber (a chamber spacer that incorporates a valve, so that aerosol is retained within the chamber for a finite time after pMDI actuation) is a convenient and cost-effective option and provides efficacy similar to that achieved with a jet nebulizer.62
In general, repeated administration of short-acting β-agonists at frequent intervals is recommended for relief of symptoms in patients with acute asthma.50,63 There is a dose–response relationship with increasing doses of β-agonists resulting in increasing bronchodilation.64,65 Rodrigo and Rodrigo, however, noted that 2.4 mg of albuterol every hour (400 mcg every 10 minutes) delivered via a pMDI/holding chamber produced clinically significant bronchodilation, and higher doses increased the incidence of adverse effects without enhancing bronchodilation.66,67 Other investigators have concluded that the cumulative dose, and not the dosage regimen, influences the bronchodilator response (i.e., the same response was obtained when 5 mg was given as a single dose as when it was given as two doses of 2.5 mg).68,69 The usual dose necessary to produce bronchodilation in severe asthma is between 5 and 10 mg of albuterol.70 Use of higher doses during the initial phase of an acute exacerbation of asthma may have clinical utility. A faster response may be seen, and, because most patients respond to 5 to 10 mg of albuterol, nonresponders could be identified. Nonresponders, defined as those requiring admission, not discharge, to an emergency department, were found to have a flatter dose–response curve to albuterol, with peak expiratory flow remaining below 45% despite administration of high doses of albuterol.68,71,72
The logistical problem in repeatedly administering doses of nebulized albuterol (intermittent therapy) stimulated interest in continuous nebulization. Various investigators have reported conflicting findings with continuous nebulization.73,74 Two meta-analysis of published trials also reached different conclusions.75,76 Overall, it appears that patients with more severe airway obstruction could benefit from continuous nebulization,4,77 especially if they do not respond to therapy within the first hour in the emergency department.
Both formoterol and arformoterol are effective for treatment of patients with COPD.78–81 There is not much published experience with their use during acute exacerbations of asthma or COPD.
In summary, the severity of an individual episode is better measured as response to bronchodilator therapy rather than by baseline pulmonary function. Continuous nebulization may be employed in patients with acute severe asthma in whom pulmonary function does not improve by the end of the first hour of intensive bronchodilator therapy.
In clinical studies evaluating bronchodilator administration through pMDI/holding chamber versus nebulizers, equivalent changes in FEV1 were produced by 6 mg/hour of albuterol given by the nebulized route and 2.4 mg/hour of albuterol given by pMDI/holding chamber, an equivalent dose ratio of 2.5:1. A higher incidence of tremor and anxiety and higher serum albuterol was seen in the nebulizer group.82 Improvement in pulmonary function is similar70,83,84 or slightly better85,86 with pMDI/holding chamber than with nebulizers, while increases in heart rate are more frequent with nebulizers.70
The ideal bronchodilator and optimal regimen for acute exacerbations of COPD have not been established. A pooled analysis comparing the effects of β-agonists (fenoterol and metaproterenol) and anticholinergics (ipratropium) found no significant differences in improvement in FEV1.87 If response to β-agonists is suboptimal or the exacerbation is severe, ipratropium can be added.88
A short course of systemic corticosteroids may be necessary to gain control of asthma symptoms. ICS may be started at the same time and the systemic steroid tapered slowly. Subsequent adjustments in ICS dose are based on assessments of symptoms, rescue use of short-acting β-agonists, and measurement of peak expiratory flow rates.
High-dose ICSs administered by pMDI to patients with acute asthma exacerbations achieve similar improvements in symptoms and pulmonary function as systemically administered corticosteroids.89–91
In patients with COPD, regular use of ICSs produces a small increase in pulmonary function and improves health status.92–94 ICSs, however, do not alter the rate of decline in FEV1 and they do not reduce mortality.95 In acute exacerbations, oral or parenteral corticosteroids are often employed with some benefit for patients who are admitted to the hospital.96,97 Current evidence favors moderate over high doses, and short courses of oral corticosteroids over intravenous therapy for treating acute exacerbations.98–100
Risk-to-benefit considerations have prompted investigation of ICS use for treatment of acute exacerbations of COPD. Several investigators have shown that the efficacy of nebulized budesonide is comparable to that observed with systemic corticosteroids, except in the most severely ill patients.101–104
In patients receiving long-term ventilation for severe COPD, Nava et al observed a small, but statistically significant, reduction in airway resistance with fluticasone.105 The optimal methods of administering ICS and the appropriate doses in ventilated patients have not been determined, but are likely to be higher than those used for maintenance therapy in COPD.16,101–104 Moreover, many ventilated patients receive systemic corticosteroids for acute exacerbations of asthma and COPD, and it is unclear if patients derive additional benefits from ICSs in the presence of high-dose therapy with oral or parenteral corticosteroids. Finally, regular use of ICSs in ambulatory patients with COPD is associated with a higher risk of pneumonia.60 Because ventilated patients are already vulnerable to developing pneumonia for a variety of reasons, the potential to add another risk factor by administering ICSs requires serious consideration. In summary, ICSs may have a limited role in ventilated patients, but further investigations are needed to determine the appropriate dosing regimen and risk-to-benefit ratio of using ICSs in this group of patients.
In acute asthma, some investigators found additional benefits with combined anticholinergic and β-agonist therapy over β-agonists alone,106–108 whereas others109,110 found no difference. A meta-analysis that compared albuterol alone to albuterol with ipratropium found an overall improvement of 7.3% in FEV1 and 22.1% in peak expiratory flow rates, and reduction in hospitalization rates for studies in which data were available.111 A more recent meta-analysis by Rodrigo and Rodrigo112 found benefit for a combination of anticholinergics with albuterol in severe asthma (FEV1 <50%).
In patients with acute exacerbations of COPD, adding an anticholinergic to a β-agonist may113 or may not114–116 provide greater bronchodilation. Fernandez et al117 found an improvement in airway pressures in ventilated patients with the combination of ipratropium and fenoterol in comparison to ipratropium; ipratropium alone achieved no benefit.
There is little doubt that combinations of ICSs plus long-acting β-agonists (Advair Diskus; Advair pMDI; Symbicort pMDI) are the most effective and widely used treatments currently available for treatment of asthma. A combination of formoterol and budesonide, used as a reliever as well as maintenance therapy, may be employed for treatment of mild to moderate acute asthma exacerbations that could be managed at home. This management strategy significantly reduces the frequency of more severe exacerbations.118 Currently, there is limited experience with bronchodilator and ICS combination therapy for treatment of acute exacerbations of COPD, especially in ventilated patients.
Theophylline is an inexpensive and commonly employed bronchodilator in many developing countries. The reader is referred to the second edition of this book17 for details on its pharmacology and mechanisms of action.
Theophylline administration requires empiric loading and maintenance dosing with frequent measurement of serum levels and dose adjustment.119 Plasma theophylline levels should be maintained between 5 and 15 mg/L because adverse effects frequently occur with higher levels.120–122 At concentrations less than 15 mg/L, theophylline is a relatively weak bronchodilator and its beneficial effects are more likely explained by its antiinflammatory action, particularly in synergy with corticosteroids.123
In acute asthma, theophylline does not confer additional bronchodilation in patients receiving intensive therapy with inhaled β-agonists and intravenous corticosteroids.124–126 Likewise, routine use of theophylline in acute exacerbations of COPD is not supported by randomized controlled trials.114 Theophylline may have a role in reducing hospitalization among patients receiving emergency department treatment for asthma and COPD,127 and in promoting corticosteroid activity in patients with acute exacerbations of COPD.128 Few investigators have reported on the use of theophylline in ventilated patients.117,129,130 In the neonatal ICU, theophylline is employed to prevent apneas.131 In adults, loading doses of intravenous theophylline produced significant bronchodilation, comparable to that achieved with two inhalations of either albuterol or ipratropium bromide.117,129,130 The increased respiratory muscle strength with theophylline132–134 may help in weaning.
Frequent side effects with theophylline, especially nausea and vomiting, are a major drawback. Older patients and those with a low serum albumin are particularly susceptible to serious theophylline toxicity, such as cardiac arrhythmias and seizures.135–137 In summary, the high frequency of side effects, its relatively low efficacy, frequent drug interactions, complicated dosing regimens, and the need for repeatedly monitoring serum levels have significantly limited theophylline use in the ICU.
Phosphodiesterase E4 (PDE4) is the predominant isozyme responsible for metabolizing cyclic adenosine monophosphate in airway smooth muscle, and in immune and inflammatory cells.138,139 Elevation of cyclic adenosine monophosphate by selective PDE4 inhibition has a wide variety of pharmacologic effects. Orally administered selective PDE4 inhibitors have bronchodilator and antiinflammatory effects in patients with asthma and COPD.140–145 Unlike theophylline, these agents do not exhibit significant drug interactions.146,147 Side effects, such as nausea and headache, are generally mild to moderate, but could limit the use of these agents in critically ill patients.
Leukotrienes are formed by the action of 5-lipoxygenase on cell membrane-derived arachidonic acid. Zileuton blocks 5-lipoxygenase and inhibits leukotriene synthesis, whereas zafirlukast, montelukast, and pranlukast all block the final step in the action of leukotrienes on the leukotriene receptor. The leukotrienes are mainly employed in patients with chronic asthma as adjuncts to ICSs. In acute asthma, intravenous montelukast achieves modest early gains in lung function without significant improvement in rates of hospital admission.148
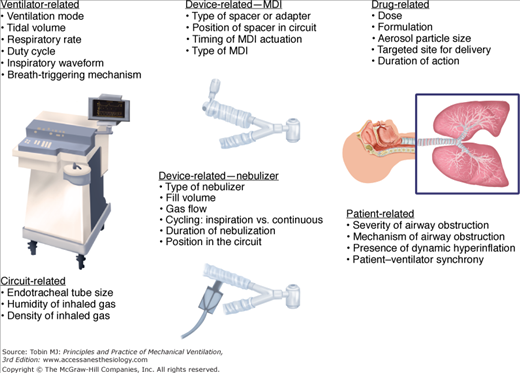
Factors Influencing Aerosol Delivery during Mechanical Ventilation
Several factors influence aerosol delivery during mechanical ventilation, including variables related to the aerosol-generating device, the ventilator and ventilator circuit, the inhaled drug or agent, and the patient (Fig. 63-1). Aerosol delivery during mechanical ventilation depends to a great extent on the type of aerosol-generating device employed.
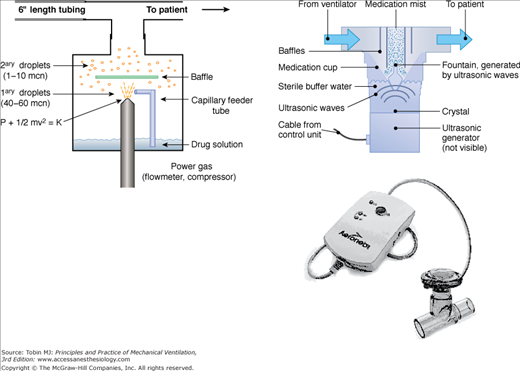
Several types of nebulizers, jet, ultrasonic, vibrating mesh, and soft-mist inhalers convert liquids into aerosols for inhalation (Fig. 63-2).61
Figure 63-2
Several types of nebulizers are available for clinical use, including jet nebulizers (left), ultrasonic nebulizers (upper right), and vibrating mesh nebulizers (lower right). The jet nebulizers employ compressed gas or air to generate an aerosol. In ultrasonic nebulizers, aerosol is generated by vibration of a piezoelectric crystal at ultrasonic frequencies. In the vibrating mesh nebulizers, aerosol is produced by high-frequency vibration of a plate with multiple apertures. Vibration of the aperture plate pushes the liquid to be nebulized through the apertures and generates a fine particle mist. mcn, micron.
Aerosol particle size is influenced by nebulizer design, solution characteristics (density, viscosity, and surface tension) and volume, gas pressure and flow, baffle design, and ratio of liquid to gas flow.149–151 Droplet size decreases when gas flow increases, whereas droplet size increases with increase in the ratio of liquid to gas flow. A certain volume of solution (dead or residual volume) cannot be nebulized. Residual volume varies from 1 to 3 mL; it can be reduced by using a nebulizer with a conical shape, improving the wetness of the plastic surfaces, and reducing the internal surface area of the nebulizer.149,151 During operation, the solution concentration increases and its temperature decreases secondary to evaporative losses.152,153 Both increased solution concentration and cooling influence nebulizer output and particle size.151,154 Significant disadvantages of jet nebulizers are the requirement for a power source, inconveniently long treatment time, need for equipment setup and cleaning, and significant variations in the performance of various nebulizers, both within the same brand and across different brands.155–157
Most ultrasonic nebulizers have a higher rate of nebulization and require a shorter time of operation than jet nebulizers (see Fig. 63-2). Generally, the aerosol particle size is larger with ultrasonic nebulizers compared to jet nebulizers. The cost and bulk of ultrasonic nebulizers and their relative inefficiency in nebulizing drug suspensions are major limitations to their use, although smaller ultrasonic nebulizers are available and have been employed during mechanical ventilation.158–162
Newer-generation nebulizers employ a vibrating mesh or plate with multiple apertures to produce an aerosol.163,164 Because the frequency of vibration of the plates is lower than that in ultrasonic nebulizers, these devices can be operated with a battery pack and or electrical source. As a result, these devices are portable and less noisy. Moreover, these devices have negligible residual volume, and this property significantly improves the drug output. The Aeroneb Pro (Aerogen Inc., Mountain View, CA) is specifically designed as an inline nebulizer (see Fig. 63-2); a breath-synchronized version of the Aeroneb Pro—the Pulmonary Drug Delivery System (PDDS, Nektar Therapeutics, San Francisco, CA)165—has been successfully employed in ventilated patients.166 The vibrating mesh nebulizers have a high rate of nebulization, and drug output is two to four times higher than with jet nebulizers.167 Unlike ultrasonic nebulizers, the temperature of the solution does not change during operation of the vibrating mesh nebulizers, and proteins and peptides can be nebulized with minimal risk of denaturation.
The intracorporeal nebulizing catheter (Aeroprobe; Trudell Medical International, London, Ontario, Canada) is a novel, investigational device that produces an aerosol in the trachea.168,169 Preliminary data suggest that lung deposition is improved with its use compared to more conventional forms of aerosol administration.170,171
The pMDI canister contains a pressurized mixture of propellants, surfactants, preservatives, flavoring agents, and active drug, the latter comprising approximately 1% of the total contents.172,173 This mixture is released from the canister through a metering valve and stem, which fits into an actuator boot.172,173 Previously, most pMDIs used chlorofluorocarbon (CFC) propellants, but a newer generation of pMDIs contain hydrofluoroalkane (HFA) propellants.174 The formulation, metering valve, and actuator design of HFA-pMDIs are different from those of CFC-pMDIs.61
The efficiency of drug delivery with a pMDI depends on how well the canister stem fits into the spacer adapter. In bench models of mechanical ventilation, HFA-pMDIs used with an AeroVent spacer provided lower drug delivery than that achieved with CFC-pMDIs.175 In contrast, beclomethasone HFA-pMDIs employed with an AeroChamber HV MV spacer (Monaghan Medical, Plattsburgh, NY) had a higher efficiency of drug delivery than the beclomethasone CFC-pMDI.176 Likewise, HFA-pMDIs were shown to achieve drug delivery similar to CFC-pMDIs in pediatric and neonatal bench models of mechanical ventilation.177,178 To improve drug delivery with HFA-pMDIs during mechanical ventilation, actuators that better fit the stem of the HFA-pMDI canister must be employed.
Dry powder inhalers could be employed inline in ventilator circuits either by employing the ventilator’s inspiratory airflow to generate an aerosol or by first producing an aerosol and then entraining the drug particles into the airflow from the ventilator. Everard et al179 employed a modified Turbuhaler in a dry ventilator circuit, and found that approximately 20% of the nominal dose was delivered to a filter placed at the distal end of the endotracheal tube. Humidity reduces drug delivery from dry powder inhalers,180 and because ventilated patients routinely receive warm and humidified gas, the feasibility of administering dry powders during mechanical ventilation needs further evaluation.
Aerosol deposition in nasal passages significantly reduces drug delivery to the lung181–183 and could reduce bronchodilator efficacy;184 however, face masks may be necessary for treatment of acutely dyspneic or uncooperative patients. For optimal efficacy, the face mask should produce a tight seal185–187 to avoid aerosol leakage and increased aerosol deposition around the eyes.188
The orientation of the nebulizer with respect to the face mask influences the pattern of aerosol deposition. In “front-loaded” masks the nebulizer is inserted directly into the face mask, whereas in “bottom-loaded” masks the aerosol enters the mask from below. Front-loaded masks provide greater inhaled mass but also produce greater facial and ocular deposition.189 Deposition of aerosol on the face and eyes could be minimized by employing masks that incorporate vents and have cut outs in the region of the eyes.189,190
Humidified high flow nasal cannulae are increasingly employed in the ICU to enhance gas exchange and avoid mechanical ventilation.191 In a bench study, the inhaled mass of aerosol and aerosol particle size with high-flow nasal cannulae were comparable to those obtained with mouthpiece inhalation from a continuously operating jet nebulizer.192
In the past, pMDIs and nebulizers193,194 were shown to have poor efficiency during mechanical ventilation, mainly because of drug deposition in the ventilator circuit and artificial airway.195,196 Both in vitro and in vivo studies have helped in understanding the complex factors governing aerosol delivery during mechanical ventilation.196–198
Whereas in vitro methods measure drug delivery to the lower respiratory tract, in vivo methods measure the amount of drug deposition in the lung. This distinction is important because a variable portion of inhaled particles do not deposit in the lung and are exhaled. A “mass balance” technique that matches ventilator circuits and ventilator parameters has been employed to determine the correlation between the results of in vitro tests and those in ventilator-supported patients.199,200 With such techniques, it was estimated that approximately 5% of the nominal dose of albuterol administered by a pMDI is exhaled by ventilated patients,175 compared to less than 1% exhaled by ambulatory patients.201 The mean exhaled fraction (7%) with nebulizers in ventilated patients is similar to that with pMDIs, but there is considerable variability (coefficient of variation: 74%) among patients.199
The particle size of the aerosol is an important determinant of aerosol delivery to the lung. Devices that produce aerosols with mass median aerodynamic diameter less than 2 μm are more efficient during mechanical ventilation than devices that produce aerosols with larger particles.199,200 Nebulizers that produce smaller particle size have been employed, but they require a considerably greater time to deliver a standard dose.200,202 Moreover, a significant proportion of submicronic particles (<1 μm) are exhaled.199 For optimal pulmonary deposition, the size of the particles in the aerosol should be small enough to allow maximum penetration through the artificial airway, yet large enough to avoid being carried back out into the atmosphere with the exhaled breath.
Carefully performed in vitro tests that simulate the conditions of actual clinical use have played an important role in determining the optimal techniques for administering aerosols to ventilated patients.175,176,199,200,202–209 Table 63-2 shows various bench models that were used. Models that employ a tracheobronchial model and directly measure the amount of drug deposited on a filter placed distal to the endotracheal tube175,176,195,209–211 have produced the most reproducible results.
| Type of model and Ref. | Type of Adapter | Breath Type | Measurement | Results |
|---|---|---|---|---|
| ETT (6.0, 7.5 and 9.0 mm) in trachea204 | Swivel adapter | Continuous flow or MDI actuation then flow | Filter weight | Greater efficiency with larger ETT and actuation into continuous flow |
| ETT and laser spectrometer205 | Three different adapters inline or cylindrical spacer | VT 800 mL; flow 60 L min-1 | Particle volume 1 to 5 μm | Adapters produced lower volume of particles than standard actuator |
| Ventilator circuit; ETT (8 mm)209 | Swivel adapter at ETT or cylindrical spacer | VT 800 mL; flow 48 L min-1 | Albuterol assay | Greater deposition with cylindrical spacer |
| ETT and laser spectrometer206 | Nine different MDI spacers or adapters | – | Particle volume 0.7 to 5.0 μm | Chamber spacers delivered greater volume than other adapters |
| Ventilator circuit207 | MDI with large chamber or small chamber spacer | VT 700 mL; flow 50 L min-1 | Radioactivity | Similar delivery with the devices |
| Plastic syringe and simulated carina210 | MDI with catheter | – | Albuterol assay | ≈90% of dose delivered beyond ETT |
| ETT (6 mm) and swivel adapter211 | Catheters placed in ETT (13 or 22 cm long) | Flow 30 L min-1 | Albuterol assay | Longer catheters delivered greater dose than shorter catheters |
| Model of trachea and main bronchi195 | Cylindrical spacer 8 mm ETT | Flow 40 L min-1 | Albuterol assay | Decreased deposition with humidification and CMV breaths |
For any given aerosol-generating device, the efficiency of drug delivered varies widely; for pMDIs, it varies from 0.3% to 97.5% and for nebulizers from 0% to 42%. These variations in drug delivery underscore the need for optimizing the techniques of administration with each device. With pMDIs, the type of pMDI propellant formulation176 and the drug formulation203 also influence drug delivery.
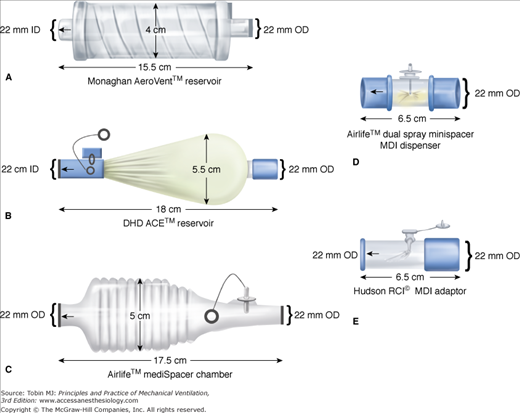
For a pMDI to be employed in a ventilator circuit, the canister must be removed from the actuator (supplied by the manufacturer) and connected to the ventilator circuit with another different adapter, thereby making it a unique device with different aerosol characteristics and performance. Several types of adapters, including elbow adapters, inline devices that may be unidirectional or bidirectional, and chamber or reservoir adapters, are commercially available (Fig. 63-3).162,212 The adapter efficiency could have a significant influence on the dose required to produce a therapeutic effect.213 Several investigators have shown that employing a chamber spacer with a pMDI in a ventilator circuit results in fourfold to sixfold greater aerosol drug delivery compared with either an elbow adapter or a unidirectional inline spacer.202,205,209,214 A pMDI and chamber spacer placed at a distance of approximately 15 cm from the endotracheal tube provides efficient aerosol delivery and elicits a significant bronchodilator response.12,197,215 The efficiency of a bidirectional inline spacer was higher than that of a unidirectional inline spacer203 and was comparable to that achieved with chamber spacers,203 although the performance of the bidirectional spacer has not been established in clinical studies.
Figure 63-3
Commercially available spacers and adapters that are used to connect a metered-dose inhaler (MDI) canister in the ventilator circuit. Top left: Collapsible spacer chamber. Middle left: Aerosol cloud enhancer, wherein the aerosol flume is directed away from the patient. Bottom left: Noncollapsible spacer chamber. Top right: Bidirectional actuator (mini spacer). Bottom right: Inline adapter. OD, outside diameter. (Used, with permission, from Rau et al.203)
Both jet and ultrasonic nebulizers are connected in the inspiratory limb of the ventilator circuit or at the patient Y. Placing a jet nebulizer at a distance from the endotracheal tube improves its efficiency compared with placing it between the patient Y and endotracheal tube.208,216,217,218 Placement of the continuously operating jet nebulizer before the humidifier (i.e., between the humidifier and ventilator) had a higher efficiency of aerosol delivery than placement closer to the endotracheal tube,218 probably because the inspiratory limb of the ventilator circuit acts as a reservoir for the aerosol during the exhalation phase. For the same reason, addition of a reservoir between the nebulizer and endotracheal tube also modestly increases efficiency of drug delivery.219 The nebulizer brand,157,208 diluent volume, operating pressures and flows, and duration of treatment,149,157 influence the efficiency of aerosol generation.
Ultrasonic nebulizers are infrequently employed for bronchodilator therapy during mechanical ventilation and there is scant published information about drug delivery with these devices.160 Moreover, the particle size of aerosols produced by ultrasonic nebulizers in ventilator circuits has not been well characterized. The newer vibrating mesh nebulizers deliver twofold to fourfold higher drug dose than jet nebulizers. In the absence of bias flow, a vibrating mesh nebulizer was most efficient for drug delivery when it was placed 15 cm from the endotracheal tube, whereas in the presence of bias flow (2 L/min) the efficiency of the device was enhanced by placing it at the inlet of the humidifier (i.e., between the humidifier and ventilator).218
The actuation of a pMDI must be synchronized with the precise onset of inspiratory airflow from the ventilator.220,221 As short as a 1- to 1.5-second delay between pMDI actuation and a ventilator breath can profoundly reduce the efficiency of drug delivery.202
In a ventilator circuit, nebulizers can be operated continuously or intermittently by airflow from the ventilator. Continuous aerosol generation requires a pressurized source of gas (from a wall outlet, pressurized tank, or an air compressor), whereas intermittent operation that is synchronized with inspiratory airflow requires a separate line to conduct inspiratory airflow from the ventilator to the nebulizer. Intermittent operation of the nebulizer is more efficient for aerosol delivery compared with continuous aerosol generation because it minimizes aerosol wastage during the exhalation phase of the breathing cycle.200,217 The lower driving pressure provided by the ventilator (<15 pounds per square inch [psi]) than that provided by pressurized gas (≥50 psi) could decrease the efficiency of some nebulizers.222 Aerosol generated by a nebulizer operating at the lower pressure may generate particles whose diameter is larger than the 1 to 5 μm that is optimal for aerosol deposition. When intermittent nebulizer operation is employed, the specific ventilator and nebulizer brand should be tested to determine the characteristics of the aerosol generated and the efficiency of drug delivery.200
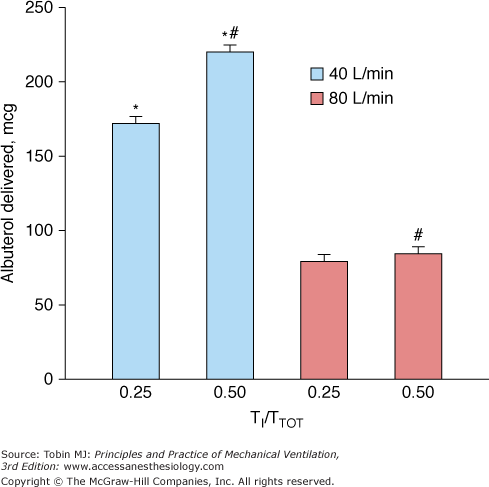
The characteristics of the ventilator breath have an important influence on aerosol drug delivery. A tidal volume of 500 mL or more (in an adult),195 longer inspiratory time, and slower inspiratory flows improve aerosol delivery195,223,224 (Fig. 63-4). An inspiratory flow rate of 30 to 50 L/min is optimal for aerosol delivery; however, slow inspiratory flow rates may increase inspiratory time and by reducing the time for exhalation may have the unintended consequence of increasing auto–positive end-expiratory pressure.225 Drug delivery is linearly correlated with a longer duty cycle (inspiratory time-to-total time [TI/TTOT]) for both pMDIs and nebulizers.175,195,208 Moreover, drug delivery is improved when a pMDI is synchronized with a simulated spontaneous breath compared with a controlled ventilator breath of similar tidal volume.
Figure 63-4
Comparison of aerosol delivery at different inspiratory airflows and duty cycles (TI/TTOT) in a bench model of mechanical ventilation. The ventilator delivered a tidal volume of 1000 mL with a constant inspiratory flow of 40 or 80 L/min, and the frequency of breathing was varied to achieve TI/TTOT values of 0.25 or 0.50 at each inspiratory flow setting. Albuterol delivery to the bronchi was greater with a TI/TTOT of 0.50 than of 0.25 at inspiratory flows of 40 L/min and 80 L/min. For each value of TI/TTOT, drug delivery with a slower inspiratory airflow (40 L/min) was almost twice that at the faster inspiratory airflow (80 L/min). *,p < 0.01, 40 L/min versus 80 L/min at TI/TTOT of 0.25 and TI/TTOT of 0.50; #, p <0.01 TI/TTOT of 0.5 versus TI/TTOT of 0.25 at 40 L/min and 80 L/min. (Dhand R. Aerosol delivery during mechanical ventilation: from basic techniques to new devices. J Aerosol Med Pulm Drug Deliv. 2008;21:45–60.)
The inspiratory waveform influences drug delivery from nebulizers, but has much less influence on drug delivery from a pMDI.226 Unlike pMDIs, nebulizer efficiency could be different during pressure-controlled ventilation than during volume-controlled ventilation (Fig. 63-5).
Figure 63-5
Comparison of aerosol delivery from a pressurized metered-dose inhaler (pMDI) and jet nebulizer in bench models of pressure-controlled and volume-controlled ventilation. The lung mechanics were varied by selecting two settings of resistance and compliance to achieve high or low time constants. For each condition, the amounts of aerosol delivered during inspiratory times of 1 second or 2 seconds were measured. Increasing the duration of inspiration from 1 second to 2 seconds improved the nebulizer efficiency. In the high-compliance/high-resistance setting with 1-second inspiration, nebulizer efficiency was higher during pressure-controlled than during volume-controlled ventilation, whereas the converse occurred in the low-compliance/low-resistance setting with 2 seconds inspiratory time. In contrast, the efficiency of a pressurized MDI (horizontal purple area) remained fairly constant under the various conditions simulated in the bench model. Thus, several factors, such as inspiratory time, pattern of inspiratory flow, and lung mechanics, that could influence drug delivery from a nebulizer have minimal influence on drug delivery from a pMDI. PCV, pressure-controlled ventilation; VCV-C, volume-controlled ventilation with a constant inspiratory flow; VCV-R, volume-controlled ventilation with a descending ramp flow pattern. (With kind permission from Springer Science and Business Media: Hess DR, Dillman C, Kacmarek RM. In vitro evaluation of aerosol bronchodilator delivery during mechanical ventilation: pressure-control vs. volume control ventilation. Intensive Care Med. 2003;29:1145–1150.)
The breath-triggering mechanism does not significantly influence drug delivery from a pMDI, but use of a flow trigger with a nebulizer could dilute the aerosol and increase the washout of the aerosol into the expiratory limb between breaths.195,208
Several investigators found that drug delivery to the lower respiratory tract from both pMDIs and nebulizers is reduced by 40% or more in a humidified circuit when compared to a dry circuit (Fig. 63-6).158,175,200,202,207,208 Circuit humidity increases the size of drug particles generated by a nebulizer.227 When a pMDI is employed in a ventilator circuit, humidity probably interferes with propellant evaporation so that drug particles remain of a larger size and impaction losses are increased.212,228
Figure 63-6
Effect of humidity on aerosol delivery. The delivery of aerosol to the lower respiratory tract in bench models of mechanical ventilation is reduced by approximately 40% when the circuit is humidified instead of dry. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Studies: O’Riordan et al. 1994, Fuller et al. 1992, Diot et al. 1995, and Fink et al. 1996, (Reprinted with permission of the American Thoracic Society. Copyright © 2012 American Thoracic Society. From Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med. 1997;156:3–10. Official Journal of the American Thoracic Society.)
Heat and moisture exchangers (HMEs) are employed as an alternative to heated humidifiers.229 The filter in the HME captures the heat and moisture in the exhaled breath and transfers part of it to the air in the following inspiration. The HME filter captures drug particles in the aerosol and markedly reduces the efficiency of drug delivery. Therefore, the HME must be removed from the circuit during aerosol treatments. Although placement of a nebulizer between the HME and endotracheal tube could provide adequate drug delivery, backflow of aerosol from the nebulizer could deposit on the HME filter, increase its airflow resistance, and increase the work of breathing for the patient.230
The density of the inhaled gas also influences drug delivery. High inspiratory flows employed during mechanical ventilation are associated with turbulence. Inhalation of a less-dense gas, such as a helium-oxygen 70/30 mixture, makes airflow less turbulent and more laminar. The use of helium-oxygen mixtures improved aerosol drug delivery in a pediatric model of mechanical ventilation.231 In a bench model of adult mechanical ventilation, drug delivery from a pMDI was noted to be 50% higher with a helium-oxygen 80/20 mixture than with oxygen alone (Fig. 63-7A).232 In contrast, nebulizer operation with helium-oxygen reduced drug output and respirable mass (Fig. 63-7B).232,233 A practical method to achieve maximum pulmonary deposition of aerosol from a nebulizer during mechanical ventilation is to operate the nebulizer with oxygen at a flow rate of 6 to 8 L/min and to entrain the aerosol generated into a ventilator circuit containing helium-oxygen (Fig. 63-8).233 Before employing helium-oxygen in ventilated patients, it is important to ensure that the ventilator is compatible with the use of such gas mixtures.234