LUNG SOUNDS
Clinicians are exhorted to always place their stethoscopes directly on a patient’s skin. Yet, when patients are examined in hallways and prehospital settings or in locations where cultural norms prevent patients from disrobing, this rule is often violated. That is not a problem: By applying pressure on the stethoscope head, clinicians can hear all the sounds normally heard on bare skin, through up to two layers of indoor clothing—including double-layered flannel shirts. Of course, inspection and percussion cannot be done through clothing, and clothing-induced acoustic artifacts may create problems.1
QUANTIFYING PLEURAL FLUID USING ULTRASOUND
Estimate the amount of pleural fluid using an ultrasound examination on supine patients. Elevate the chest to 15 degrees and move the probe perpendicular to the body axis along the posterior axillary line. Measure the maximal pleural separation, which is usually visible at the lung base. The simplified formula is: Volume of pleural fluid (mL) = 20 × Maximal distance between parietal and visceral pleura in end expiration (mm).2
PULMONARY TREATMENT
The use of aerosol spacers more than doubles the amount of medication delivered to the lungs from metered-dose inhalers (MDIs); for steroid inhalers, an aerosol spacer diminishes the incidence of oral candidiasis by decreasing deposition in the oropharynx. The tube from a roll of toilet paper works well as an aerosol spacer, as does a piece of ventilator tubing.
Dr. Lara Zibners-Lohr wrote that in remote areas of the world, she uses large Styrofoam cups. The cup lip (open end) goes over the nose and mouth. The MDI goes through a hole in the bottom end of the cup. She uses just the blue tubing from a nebulizer for older children: they close their mouth around one end and put the MDI in the other. (Personal written communication, June 5, 2007.)
Dr. Karen Schneider uses a dry water bottle with a hole in the bottom for the MDI. The hole is sealed with tape, leaving a small opening to allow air movement from the outside when the child inhales. The inside of the bottle must be dry; otherwise, the aerosolized particles will stick to the water. When the MDI is activated, the child places his or her mouth over the drinking end and inhales a few times until the mist is cleared (Fig. 9-1). It is important that the child not exhale into the bottle because this will blow the mist out the small hole. (Written communication, June 5, 2007.)
Improvised spacers have been shown to be just as effective as the expensive commercial spacers.3
An unremitting cough may be due to airway hyperirritability caused by an upper respiratory infection, toxic inhalation, asthma (sometimes unrecognized), allergens, or the use of angiotensin-converting enzyme (ACE) inhibitors. The cough is uncomfortable for the patient and may worsen bronchospasm. For adults and children, add 0.5 mg/kg of lidocaine to 0.3 mL of albuterol solution in 3 mL of normal saline; administer the combination by aerosol nebulization. Lidocaine suppresses the cough reflex while the sympathomimetic agent relieves the bronchospasm.4
In patients with unilateral lung disease, positioning the healthy lung down—in the most dependent position possible—may improve the ventilation–perfusion mismatch and raise oxygen saturation levels. This may buy valuable time, turning an emergent situation into an urgent one.5
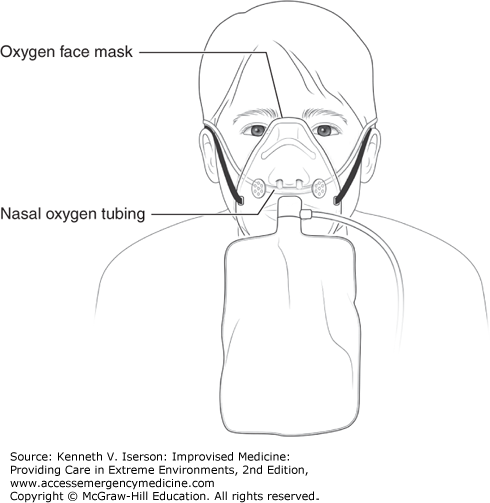
The FiO2 can be doubled by combining nasal oxygen (15 liters/minute) and non-rebreather face mask oxygen (15 L/min) (Fig. 9-2). The technique delivers close to 100% FiO2 (rather than the typical 60%) by eliminating the accumulation and rebreathing of CO2 in the mask, hypopharynx, and nasopharynx. This method is useful to buy time to set up formal noninvasive ventilation (NIV) equipment or to more quickly preoxygenate before intubation. If using a bag-valve-mask (BVM), attach a positive end-expiratory pressure (PEEP) valve (set the dial at 5 mm Hg) to produce a slightly positive end-expiratory pressure through the ventilation cycle.6
Under anesthesia, and probably in other critical care situations, morbidly obese patients have a wide alveolar–arterial oxygen gradient [P(A-a)O2]. However, when they are placed in the reverse Trendelenburg position (RTP), with their head higher than their feet, their A-a gradient shows a significant improvement and a return toward baseline. In addition, total respiratory system compliance is significantly higher in RTP, suggesting that RTP is an appropriate position for obese subjects who can tolerate it, because it causes minimal arterial blood pressure changes and improves oxygenation.7
Sitting a ventilated patient up also helps avoid gastric aspiration. Use this simple maneuver in any patient whose blood pressure can tolerate a semi-recumbent or upright position.8
Clinicians now commonly use noninvasive ventilation to avoid or delay intubating patients.
Guidelines for Appropriate Use Outside Critical Care Areas (from University Medical Center, Tucson, Ariz., 2014)
Stable patients who require NIV for obesity hypoventilation syndrome, obstructive sleep apnea, or other chronic, stable conditions. The patient can go to an unmonitored inpatient bed.
Palliative care use of NIV to alleviate suffering at the end of life. The patient can go to an unmonitored inpatient bed.
Acute or acute-on-chronic respiratory distress, including patients listed under #1, above, with progressive or new problems. The patient must go to a monitored inpatient bed.
A meta-analysis suggests that early use of NIV in chest trauma patients may reduce mortality and the intubation rate without increasing complications. However, the patient selection criteria and timing for NIV remain unclear.9
Bi-level positive airway pressure (BiPAP) is becoming an increasingly useful modality to avoid intubations. In settings with ventilators but no BiPAP equipment, BiPAP can be improvised. For example, after Hurricane Katrina, Peter DeBlieux, MD, FACEP, reported that physicians at Charity Hospital improvised BiPAP machines from mechanical ventilators: “We used a pressure-cycled ventilator with pressure support of 10 cm H2O and PEEP [positive end-expiratory pressure] of 5 cm H2O and attached it to the circuit. [However,] ventilators do not tolerate the leak associated with noninvasive ventilation (NIV or NIPPV) and the alarms sound frequently with mouth opening and poor mask fit. A full-face mask is necessary when using this system, rather than a smaller nasal mask. Using BiPAP as invasive ventilators is not the standard, but could be used in a pinch for pressure supported ventilation.” (Written communication, February 2008.)
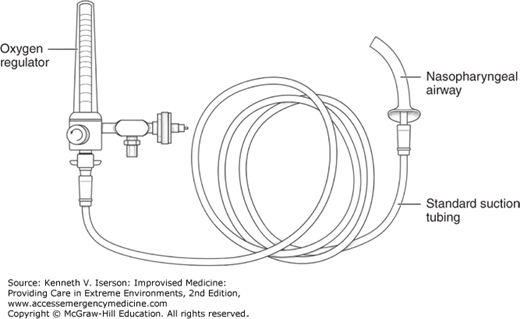
Use a nasopharyngeal airway (NPA) to generate positive-pressure oxygenation when other methods will not work. This method can provide NIV in patients with a poor mask seal and apneic oxygenation during procedures, including intubation. It also allows positive-pressure oxygenation without obstructing airway visualization. To use this method, insert an NPA into the nostril and connect it, using standard wall suction tubing, to an oxygen regulator opened to >25 L/min, if the regulator goes that high (Fig. 9-3). Excessive pressure exits through the open nare and mouth. Aspiration and gastric insufflations are the primary risks, as with other NIV methods.10
In neonates and infants, an infant feeding tube (IFT) can deliver continuous positive airway pressure (CPAP) simply, economically, and with fewer adverse effects than with traditional methods. Insert an IFT into the nasopharynx, approximately the distance between the tip of the nose and the tragus. Connect the tube to an oxygen flow meter (usually 2-6 L/min) with a humidifier. This method is used in many developing countries with few complications (e.g., nasal mucosal injury, stomach distension, aspiration, hyperinflation, and pulmonary air leaks) that can be avoided with humidification and gastric decompression.11
MAKESHIFT SPIROMETERS
To encourage patients to breathe deeply after surgery, trauma, or illness, use an incentive spirometer, such as a balloon or a surgical glove. Have the patient blow one up several times an hour while awake. It works, however, only if the patient has received adequate analgesia.12
Incentive spirometers can also be made from plastic bottles, such as those for milk or soda. Fill the bottle about halfway with water. Insert a tube with an internal diameter (ID) of at least 0.5 cm so that the resistance is not too great (Fig. 9-4). Insert the tube into the bottle’s open mouth; this can be messy if the bottle tips over. Instead, you can insert the tube through a hole drilled in the resealed cap, although, in that case, either the cap must be left loose or a small additional hole must be made so that air can escape. Adjust the water height (resistance) for the patient; less water may be necessary for children and for the elderly and infirm. Have the patient blow bubbles. Coloring the water with food coloring makes it more fun for children. Spirometers improvised to measure lung function produce unreliable measurements.
OXYGEN
Oxygen is one of the most basic drugs we have. In many acute illnesses, such as acute respiratory infections, asthma, fetal asphyxia, and shock, the availability of an oxygen supply can save a patient’s life. It becomes especially important during resuscitations, in the operating room, or when treating cardiopulmonary illnesses and any illness (including acute mountain sickness) at altitude.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree