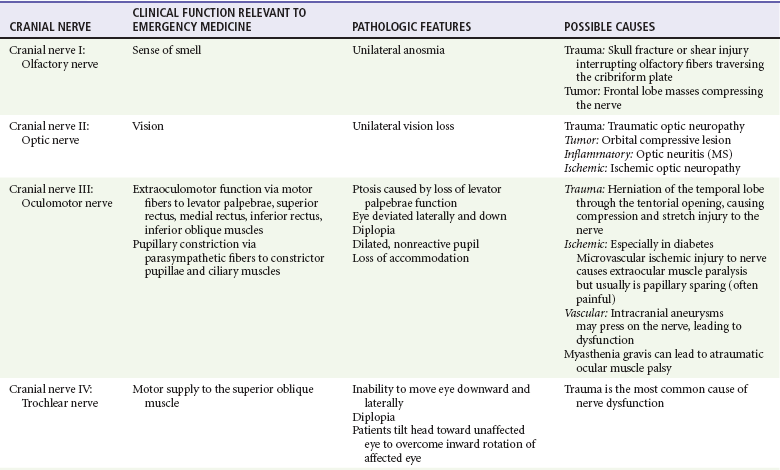
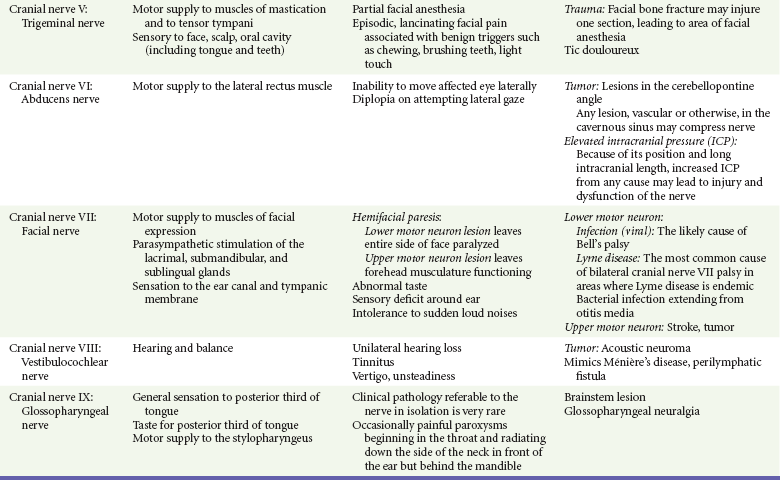
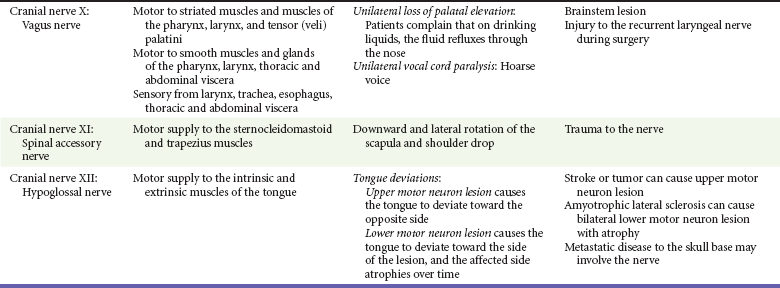
Chapter 105 This chapter discusses cranial nerve problems, cerebral venous thrombosis, and multiple sclerosis—neurologic disorders that often provide significant diagnostic and therapeutic challenges in the emergency department (ED) setting (Table 105-1). Trigeminal neuralgia, or tic douloureux, is a syndrome featuring painful paroxysms in one or more distributions of the trigeminal nerve. Trigeminal neuralgia is relatively uncommon, with an annual incidence of 4 to 13 cases per 100,000 population.1,2 It is more common in women than in men, with a female-to-male ratio of 1.7 : 1. Affected persons typically are between 50 and 69 years of age, and symptoms occur more frequently on the right side of the face.3 Trigeminal neuralgia is an idiopathic disorder, although significant evidence points to vascular compression of the trigeminal nerve root in many cases. This compression commonly is caused by a tortuous arterial or venous loop in the posterior fossa, an arteriovenous malformation, or rarely a tumor. In surgical case series, vascular compression of the trigeminal nerve root is found in 80 to 90% of cases.4,5 Of note, however, structural lesions are not found in all patients with trigeminal neuralgia.6 Trigeminal neuralgia is manifested with unilateral facial pain, typically characterized as lancinating paroxysms of pain in the lips, teeth, gums, or chin. The pain of trigeminal neuralgia is commonly associated with physical triggers such as chewing, brushing the teeth, shaving, washing or touching the affected area of the face, swallowing, or exposure to hot or cold temperature in the affected area. The maxillary and mandibular divisions of the trigeminal nerve are most commonly involved; rarely, the ophthalmic division alone is involved. Patients tend to experience the pain in clustered episodes that last a few seconds to several minutes. The attacks can occur during the day or night but rarely arise during sleep.6,7 A careful history and physical examination should be performed to rule out other painful facial conditions, including odontogenic infections, sinus disease, otitis media, acute glaucoma, temporomandibular joint disease, and herpes zoster. Patients who lack local pathologic findings to explain the painful syndrome require a careful neurologic examination. The presence of a neurologic deficit should prompt suspicion of a structural lesion, such as aneurysm, tumor, or other intracranial lesion (e.g., from multiple sclerosis). Of note, 2 to 4% of patients with trigeminal neuralgia also have multiple sclerosis.8 Patients with normal findings on the head and neck examination and no neurologic deficits who have episodic, unilateral facial pain associated with nonpainful triggers are likely to have trigeminal neuralgia. Since the 1960s the treatment of choice for trigeminal neuralgia has been the anticonvulsant medication carbamazepine. The purported effectiveness of this treatment, however, is based on uncontrolled studies, and the mechanism of action of anticonvulsant therapy for trigeminal neuralgia is unclear. The true efficacy of medical therapy is difficult to assess owing to a high rate of spontaneous remission. Nonetheless, carbamazepine appears to be an effective and well-tolerated agent for treatment of trigeminal neuralgia. The initial dosage of carbamazepine is 100 mg twice daily; this dose is then increased to three times daily after 1 week. The dose may be increased by 100 mg/day, up to a maximum of 1200 mg/day. A complete blood count and liver function studies are performed periodically in patients who are taking carbamazepine to monitor for hematologic and hepatic side effects. Additional agents that have been used for treatment of trigeminal neuralgia include phenytoin, baclofen, valproate sodium, lamotrigine, gabapentin, and levetiracetam. None of these drugs has been shown to be more effective than carbamazepine.7 Central procedures can be divided into percutaneous approaches and open approaches. Percutaneous destruction of the trigeminal ganglion can be done by means of radiofrequency ablation, thermal ablation, glycerol injection, or balloon microcompression. These procedures carry the risk of corneal anesthesia, oculomotor paresis, or masticatory weakness.9 Open surgical management is the surgical option of choice in many treatment centers. Open surgical procedures include microvascular decompression of the nerve with or without partial ablation. Although the open microvascular decompression procedure has proved to be effective, with pain relief achieved in 80 to 95% of patients, the surgery is associated with the risk of significant complications, including hearing loss, facial anesthesia, cerebrospinal fluid (CSF) leak, brainstem or cerebellar injury, headaches, meningitis, and death.10,11 Gamma Knife radiosurgery, a minimally invasive, precision-directed stereotactic radiosurgery, also has been associated with good outcomes. This highly specialized technique requires extremely sophisticated stereotactic radiofrequency equipment and is available only in specialized centers, but in many cases it has similar results for pain relief without the morbidity associated with open procedures in patients who might otherwise be a surgical risk.12–14 The acute onset of facial nerve paralysis often will prompt an ED visit. Fortunately, early diagnosis and therapy may improve the patient’s chance for recovery of function of the facial nerve. Facial nerve paralysis of acute onset affects approximately 20 to 25 persons per 100,000 population per year, without geographic, gender, or race predilection.15,16 The facial nerve innervates the muscles of facial expression and the muscles of the scalp and external ear in addition to the buccinator, platysma, stapedius, stylohyoid, and posterior belly of the digastric muscles. The sensory portion of the nerve supplies the anterior two thirds of the tongue with taste and sensation to portions of the external auditory meatus, soft palate, and adjacent pharynx. The parasympathetic portion supplies secretomotor fibers for the submandibular, sublingual, lacrimal, nasal, and palatine glands.17 The nerve originates from the pontomedullary junction of the brainstem and enters the internal auditory meatus with cranial nerve VIII. Within the temporal bone, the facial nerve has four major branches: the greater and lesser superficial petrosal nerves, the nerve to the stapedius muscle, and the chorda tympani. The facial nerve exits the temporal bone at the stylomastoid foramen and then enters the parotid gland, where it divides to supply the muscles of facial expression.17,18 Bell’s Palsy.: Bell’s palsy, also commonly called idiopathic facial paralysis, has long been postulated to have a viral cause. This disease entity is characterized by an abrupt onset of a lower motor neuron paresis that can progress during 1 to 7 days to complete paralysis. A prodromal illness is described by 60% of patients. Symptoms and signs frequently associated with the facial paresis include ear pain, perception of sensory change on the involved side of the face, decreased tearing, overflow of tears on the cheek (epiphora), abnormally acute hearing (hyperacusis), and impairment or perversion of taste (dysgeusia).19 Available evidence now strongly favors the use of corticosteroids for the treatment of Bell’s palsy, and earlier treatment is associated with better efficacy and outcomes.20–24 The rationale for this application of steroid therapy is that edema of the nerve, confined within the facial canal, is thought to cause or contribute to the nerve injury. On the basis of this theory, most experts currently recommend a course of prednisone with an initial dose of 1 mg/kg per day for 7 to 10 days, with or without a short taper.15,18,25,26 The most definitive randomized, double-blind, placebo-controlled trial involving 496 patients showed an improvement in complete recovery of facial nerve function at 3 months from 64% with placebo to 83% with the use of prednisolone in a dose of 25 mg by mouth twice daily.27 Therapy should be started as soon as possible, ideally within the first 24 hours, but it is still recommended for patients without contraindications who seek treatment within 1 week of symptom onset.25 A number of studies have supported the contention that Bell’s palsy may be caused by herpes virus infection. One study demonstrated herpes simplex virus type 1 DNA in the endoneurial tissue of 11 of 14 patients with Bell’s palsy but not in control subjects.28 In a trial of prednisone and acyclovir in 99 patients, patients treated with prednisone and acyclovir had a more favorable recovery than that observed in patients receiving prednisone alone.29 A study of 296 patients with Bell’s palsy treated with valacyclovir or placebo in addition to a fixed dose of prednisolone found significant benefit to the addition of valacyclovir, particularly in the setting of severe palsy or in those treated within 24 hours of symptom onset.30 Other studies have found conflicting results. Despite a lack of overwhelming evidence, the addition of an antiviral agent should be considered in the treatment of Bell’s palsy, especially with severe loss of function. The most commonly recommended antiviral regimen is valacyclovir, 1000 mg orally two times daily for 10 days. Valacyclovir and famciclovir have better oral absorption, are better tolerated, and are dosed less frequently, resulting in higher compliance than with acyclovir treatment.18,25,26,28,31 As with steroid therapy, although earlier treatment is preferred, treatment should be considered for patients presenting within 1 week of symptom onset. Ramsay Hunt Syndrome.: Ramsay Hunt syndrome (herpes zoster oticus) is characterized by unilateral facial paralysis, a herpetiform vesicular eruption, and vestibulocochlear dysfunction. The vesicular eruption may occur on the pinna, external auditory canal, tympanic membrane, soft palate, oral cavity, face, and neck as far down as the shoulder. The pain is considerably more severe than that associated with Bell’s palsy, and it frequently is out of proportion to physical findings. In addition, outcomes are worse than with Bell’s palsy, with a lower incidence of complete facial recovery and the possibility of sensorineural hearing loss. Therapy is similar to that for Bell’s palsy. Both prednisone and antiviral therapy for 7 to 10 days are advocated.18,32,33 Lyme Disease.: Lyme disease is the most frequent vector-borne infection in the United States. It is caused by the spirochete Borrelia burgdorferi and is spread by the bite of Ixodes genus ticks. Neurologic manifestations can arise in any phase of the disease, and the incidence of facial palsy in patients with neurologic involvement is 35 to 51%. In regions in which Lyme disease is endemic, it has been shown to be the leading cause of facial paralysis in children, responsible for half of all pediatric cases of facial nerve paralysis.34,35 Bilateral facial nerve paralysis is rare but can occur with systemic infections. The two diseases most commonly associated with bilateral simultaneous onset of facial paralysis are Lyme disease and infectious mononucleosis. Bilateral facial paralysis should be considered to be a manifestation of Lyme disease until further testing excludes this diagnosis.26,34–36 The evaluation and treatment of Lyme disease are discussed in Chapter 134. Bacterial Infections.: Facial paralysis can be caused by acute bacterial infections of the middle ear, mastoid, or external auditory canal. In the pre-antibiotic era, facial paralysis was associated with acute otitis media in approximately 2% of cases; today, however, it occurs in only 0.2% of cases. Treatment consists of intravenous antibiotics and myringotomy for decompression. Malignant otitis externa can be associated with facial paralysis. This disease entity is most commonly seen in immunocompromised patients and usually is caused by Pseudomonas infection. Treatment involves prolonged intravenous antipseudomonal antibiotic therapy and may require surgical débridement.26,37 Tumors of the facial nerve itself or tumors anywhere along the course of the facial nerve that invade or compress the nerve may lead to facial paralysis. The course is typically progressive for at least 3 weeks. A sudden onset of paralysis, however, does not rule out an underlying tumor because facial paralysis secondary to a neoplasm is of sudden onset in approximately 25% of cases.38 A neoplastic cause should be suspected in patients who suffer from recurrent ipsilateral facial paralysis, significant pain, prolonged symptoms, or any other concomitant cranial nerve abnormality. Vestibular schwannoma, formally referred to as acoustic neuroma, is a rare but important cause of sensorineural hearing loss. The annual incidence of vestibular schwannoma is 1 case per 100,000 population, with a mean age at the time of detection of 46 to 58 years.39 The female-to-male ratio is 1.5 : 1. Vestibular schwannoma is rarely bilateral, occurring in this form in approximately 5% of cases and generally associated with type 2 neurofibromatosis. Although histologically benign, vestibular schwannoma can cause neurologic damage by direct compression on the eighth cranial nerve and the other structures in the cerebellopontine angle.40 Vestibular schwannoma arises from the Schwann cells covering the vestibular branch of the eighth cranial nerve as it passes through the internal auditory canal. The tumor may compress the cochlear (acoustic) branch of the eighth cranial nerve, causing hearing loss, tinnitus, and dysequilibrium. Continued growth of the tumor may result in compression of structures in the cerebellopontine angle, where the facial and trigeminal nerves may be compressed and damaged. Larger tumors may further encroach on the brainstem and if large enough may compress the fourth ventricle, ultimately resulting in signs of increased intracranial pressure (ICP).41 Asymmetrical sensorineural hearing loss is the hallmark of vestibular schwannoma. Up to 15% of patients with this tumor, however, will have normal results on audiometry. These patients typically have symptoms such as unilateral tinnitus, imbalance, headache, fullness in the ear, otalgia, and facial nerve weakness. Thus, patients with asymmetrical symptoms should be further evaluated for vestibular schwannoma even with normal findings on audiometry.42 Vestibular schwannomas are extremely slow-growing tumors, averaging an approximately 1-mm increase per year, although many do not grow at all on serial examinations.43 Symptom onset is therefore generally gradual. In one series of 126 cases, the average time from symptom onset to discovery of a vestibular schwannoma was approximately 4 years.44 When vestibular schwannoma is suspected, the patient is evaluated by audiometry or gadolinium-enhanced MRI. This imaging technique is extremely sensitive and has led to earlier diagnosis and a decrease in mean size at detection of vestibular schwannoma. CT lacks the necessary sensitivity in the posterior cranial fossa to reliably rule out the presence of vestibular schwannoma. The smaller the tumor at the time of diagnosis, the more options there are for therapy and the better the potential prognosis.40 Vestibular schwannomas account for 80% of all cerebellopontine angle tumors. Among all other lesions, meningioma is the most common. Meningiomas more frequently cause symptoms of facial palsy or trigeminal nerve abnormality. Of note, however, considerable similarity between the clinical picture of a meningioma and that of vestibular schwannoma in the cerebellopontine angle has been described.45 Vestibular schwannoma may be removed surgically or ablated with stereotactic radiation therapy. In general, tumors larger than 3 cm are recommended for microsurgery because radiation treatments, such as with the Gamma Knife or linear accelerator, are less effective for local control and growth arrest in larger masses. Smaller tumors are amenable to use of stereotactic radiation therapy, which has greater salvage rates of facial nerve function and hearing.46,47 Stereotactic radiation therapy generally has good long-term outcomes of local growth arrest, with nerve salvage approaching 90% or greater. Injuries to the trigeminal, facial, and acoustic nerves and to the cerebellum are possible complications of both procedures. In patients who are minimally symptomatic with small tumors, serial monitoring with MRI is a viable nonsurgical option. All patients should be evaluated by a specialist in the evaluation and treatment of vestibular schwannoma because smaller tumor size at detection is associated with a better long-term outcome.39,43 Cranial mononeuropathies occur uncommonly, usually are a complication of diabetes, and most often affect the extraocular muscles. The oculomotor nerve is most commonly affected, followed in order by the trochlear and abducens nerves. In one large series in Japan, the incidence of cranial nerve palsies was 1.0% among diabetics and 0.1% among nondiabetics.48,49 Whereas ophthalmoplegia appears to be closely related to diabetes, facial palsy is less strongly correlated with this disease.48,50 The pathologic basis of diabetic mononeuropathy appears to be ischemia of the affected cranial nerve caused by occlusion of an intraneural nutrient artery serving the nerve. This occlusion leads to injury located primarily in the center of the nerve because the core fibers are more dependent on the supply from such nutrient arteries. The peripheral fibers are less affected because they also are supplied by collateral vessels. In the oculomotor nerve, the preservation of the circumferentially located parasympathetic fibers explains the pupillary sparing that usually is found in this syndrome. In two studies, the microvascular changes in the intraneural arteries that lead to occlusion were noted in diabetic patients but absent in nondiabetics.51,52 Patients typically describe acute onset of unilateral retro-ocular and supraorbital pain, diplopia, and ptosis.49 The physical manifestations of a third cranial nerve palsy include the inability to move the eye superiorly and medially, accompanied by ptosis. The pupillary light reflex usually is present. Although it is a less common finding, the fourth and sixth cranial nerves may be affected. Patients with a fourth cranial nerve palsy are unable to move the eye inferolaterally, and those with a sixth cranial nerve palsy are unable to move the eye laterally. Because of the long intracranial course of the sixth nerve, a patient with an isolated sixth nerve palsy should be evaluated for an intracranial lesion or increased ICP.53
Brain and Cranial Nerve Disorders
Trigeminal Neuralgia
Pathophysiology
Clinical Features
Diagnostic Strategies
Management
Facial Nerve Paralysis
Principles of Disease
Pathophysiology
Infection
Neoplasm
Vestibular Schwannoma
Principles of Disease
Clinical Features
Diagnostic Strategies
Differential Considerations
Management
Diabetic Cranial Mononeuropathy
Principles of Disease
Clinical Features
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree