148 Botulism
Botulism is the neuroparalytic disorder resulting from intoxication with exotoxins produced by Clostridium botulinum and several other strains of clostridia. C. botulinum are spore-forming obligate anaerobic bacilli1 whose heat-resistant spores are widely distributed in soil and marine sediment throughout the world.2 The term botulism is derived from the Latin word for “sausage,” botulus. Botulism initially was recognized as sausage poisoning in Europe in the early 19th century. Kerner, a German health official, characterized the relationship between sausage ingestion and paralysis in 230 people in 1820.3 The toxin and the bacterium were initially demonstrated by Van Ermengem4 in his study of an epidemic of foodborne botulism following raw ham consumption at a Belgian funeral music festival in 1895. In addition to foodborne botulism after ingestion of preformed toxin, forms of botulism owing to in vivo toxin production subsequently were recognized, including wound botulism in 1943,5 infant botulism in 1976,6,7 and adult intestinal botulism in 1986.8 Inhalational botulism has been identified only in a single outbreak in humans9 but has received more recent attention related to the potential for aerosolized toxin used as a biological weapon.10
 Toxin Characteristics
Toxin Characteristics
Seven distinct serotypes of botulinum toxin, A through G, are defined by the absence of cross-neutralization with antitoxin.11 Human disease is produced by types A, B, E, and rarely F toxin, whereas types C and D produce disease in birds and mammals.12 Type G has been implicated in human disease only rarely.12,13 Neurotoxigenic strains of Clostridium baratii may produce type F toxin,14,15 and some strains of Clostridium butyricum may produce type E toxin.16
Botulinum toxins are 150-kD polypeptides that are converted during bacterial lysis by proteases into an active form consisting of a 50-kD light chain and a 100-kD heavy chain joined by a disulfide bond.17 After absorption into the systemic circulation, the carboxy-terminal domain of the heavy chain facilitates binding of the toxin to polysialoganglioside receptors on neuronal membranes, whereas the amino-terminal domain of the heavy chain mediates translocation of the toxin into motor or autonomic neurons.18,19 The light chain is a zinc endopeptidase that cleaves a toxin-specific location of one or more of the SNARE (soluble N-ethylmaleimide-sensitive fusion associated protein receptor) proteins mediating the docking and fusion of acetylcholine vesicles with the presynaptic membrane at the neuromuscular junction, in autonomic ganglia, and in parasympathetic nerve terminals.20 SNARE proteins, SNAP-25 (synaptosomal-associated protein of 25 kD) and syntaxin, are associated with the presynaptic membrane, whereas synaptobrevin or vesicle-associated membrane protein (VAMP) is located on the synaptic vesicle membrane. SNAP-25 is cleaved by types A, C, and E toxins, and syntaxin is cleaved by type C toxin.21–23 Synaptobrevin is cleaved by types B, D, F, and G and tetanus toxins.24–26 After cleavage of SNARE proteins by botulinum toxin, the release of acetylcholine is permanently halted at affected synapses. Recovery from botulism occurs when the presynaptic neuron sprouts another nerve terminal to reform the cholinergic synapse.27–29 The original synapse remains intact, however, and over a period of months becomes functional again. After this occurs, the new synapse is pruned.
Botulinum toxin is considered to be the most toxic substance by weight,30 with a lethal dose in humans estimated to be approximately 1 ng/kg of type A toxin.31 By extrapolation from primate studies,32 the lethal dose of type A toxin for a 70-kg man is estimated to be 70 µg by mouth, 0.70 to 0.90 µg by inhalation, and 0.09 to 0.15 µg by intramuscular or intravenous routes.33 In contrast to the heat-resistant spores of C. botulinum, the toxins are heat labile and are inactivated by heating to 85°C for at least 5 minutes.34
 Forms of Human Botulism
Forms of Human Botulism
Foodborne Botulism
Ingestion of contaminated food, with absorption of toxin from the duodenum and jejunum, causes foodborne botulism. Because several individuals may be exposed to a single contaminated food source, foodborne botulism often presents in outbreaks. The average annual number of foodborne botulism cases in the United States between 1973 and 1998 was 24 (range 14-94),35 with an average of 9.4 outbreaks a year between 1950 and 1996.12 The most frequently implicated foods include home-canned vegetables, fruits, and fish.12 Failure to use a proper combination of heat, pressure, and time to kill spores during home canning, particularly with low-acid (pH > 5) foods, may permit survival and germination of spores.36 Although restaurant and commercially prepared foods are responsible for fewer outbreaks (7% from 1950-1996),12 nearly half of foodborne cases may arise from these sources.37 Fish preparation using fermentation among Alaskan natives is responsible for a large fraction of the total cases (29% from 1973-1998).12
Foodborne botulism due to type A toxin is most common in the United States, constituting 45% of outbreaks compared with 36% of outbreaks due to type E and 13% due to type B toxin during the period 1990 to 1996. Type F foodborne outbreaks are rare in the United States.12 The geographic distribution of foodborne botulism outbreaks mirrors the type of spores residing in soil. Type A spores predominate in the western United States, and type B spores predominate in the northeastern and central United States.38,39 Type E spores are found in marine life and sediments.40,41 In a corresponding fashion, during the period 1950 to 1996, 86% of the type A outbreaks occurred west of the Mississippi River, whereas 61% of the type B outbreaks were from eastern states. Marine products have been implicated in 91% of type E outbreaks.12
Signs and symptoms of foodborne botulism generally develop within 12 to 36 hours of ingestion of contaminated food, with the acuity and severity of illness related to the amount of toxin absorbed. In general, a symmetrical, descending paralysis with multiple cranial neuropathies evolves rapidly in the absence of fever or altered sensorium. In foodborne botulism, initial symptoms are often gastrointestinal (GI) and include nausea, vomiting, diarrhea, and abdominal cramping, which may be due to ingestion of other bacterial metabolites along with botulinum toxin in contaminated food.42 Parasympathetic dysfunction may present early with dry mouth and blurred vision associated with dilated, poorly reactive pupils. Diplopia often develops secondary to extraocular muscle weakness with paretic, disconjugate eye movements. With paralysis of bulbar muscles, patients may exhibit flaccid dysarthria, chewing difficulty, and dysphagia. The upper extremities, trunk, and lower extremities may become paretic in a descending fashion. Autonomic dysfunction may manifest as GI dysmotility, orthostatic hypotension, altered resting pulse, urinary retention, or hypothermia.43
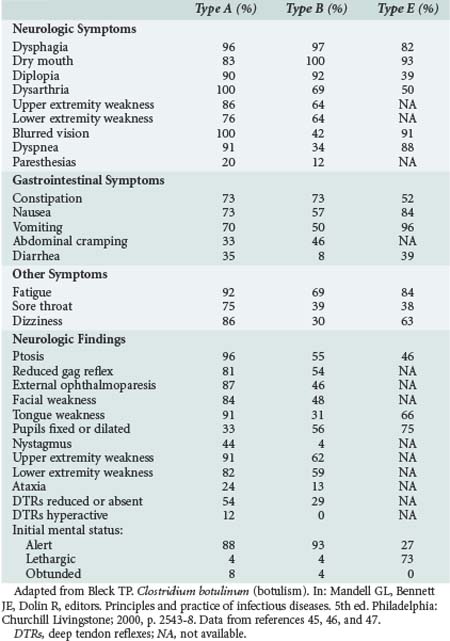
Respiratory compromise may occur secondary to a combination of upper airway obstruction from weak oropharyngeal muscles and diaphragmatic weakness. Requirements for mechanical ventilation are more prolonged for patients with type A disease (mean 58 days) compared with patients with type B disease (mean 26 days).44 The clinical findings related to intoxication with various types of botulinum toxin are varied (Table 148-1), with type A disease causing more frequent extraocular and bulbar muscle weakness, and type B and E disease causing relatively more pupillary and autonomic dysfunction.45–47
With improvements in respiratory care, the case-fatality rate has diminished from 60% during 1899 to 1949 to 12.5% during 1950 to 1996.12 The fatality risk for the index case in an outbreak is 25%, with a 4% fatality risk for subsequent cases after recognition of an outbreak.48 Because of the potential for exposure of other individuals to a contaminated food source and for additional cases accumulating from previous exposure, every case of suspected foodborne botulism should be reported to local and state public health authorities.
Wound Botulism
Wound botulism results from in vivo toxin production in abscessed and devitalized wounds.49 In the event of contamination by spores, these wounds provide an ideal anaerobic environment for spore germination and local colonization by C. botulinum with absorption of toxin into systemic circulation. In contrast to the rapid onset of botulism in foodborne disease with ingestion of preformed toxin, the incubation period for wound botulism is 7 days (range 4-14 days).50 Single cases occur in isolation with a case-fatality rate of approximately 15%.51 Before 1980, wound botulism was a rare disorder generally associated with deep wounds containing avascular areas. Between 1943 and 1985, 33 cases were reported in the United States.12 During the period 1986 through 1996, 78 cases of wound botulism were reported in the United States, most related to subcutaneous injection or “skin popping” of black tar heroin.12,52 Wound botulism due to sinusitis after repeated cocaine inhalation also has been observed.53 The neurologic signs and symptoms are virtually identical to foodborne disease except for the absence of prodromal GI symptoms.12 When present, fever is related to the wound infection.54 The diagnosis should be suspected in patients with a drug-injection history and without known exposure to a contaminated food source.55
Intestinal Botulism
Infant Intestinal Botulism
Infant and adult intestinal botulism result from the ingestion of C. botulinum spores that germinate, colonize the large intestine, and produce botulinum toxin in vivo.6 Infant intestinal botulism is now recognized as the most common form of botulism in the United States, with approximately 100 cases reported annually. About half of the cases relate to type A toxin, and the other half to type B intoxication.12 Most individual cases occur sporadically, although rare unexplained clusters are reported.56–58 Since the recognition of infant intestinal botulism in 1976, nearly half of the reported cases have occurred in California. The geographic distribution of infant botulism is unexplained, with the highest incidence rates observed in Delaware, Hawaii, Utah, and California.12 The average age of onset is 13 weeks, and most cases occur before 6 months, although some cases have occurred at 15 months of age.12
Ingestion of ambient C. botulinum spores, distributed widely in soils and dust, is thought to represent the primary route of exposure.56 Honey is also a source of spores and has been implicated as a significant risk for infant intestinal botulism.59–61 In an animal model of infant intestinal botulism, mice between 7 and 13 days old proved susceptible to intestinal colonization with C. botulinum after intragastric injection of spores.62 Epidemiologic studies suggest a parallel peak human susceptibility to intestinal colonization by C. botulinum between 2 and 4 months of age.63 This susceptibility appears related to the intestinal flora in the immature infant GI tract. The resident flora are influenced by an infant’s food sources,64 although the potential significance of breastfeeding versus formula feeding as a risk for infant intestinal botulism is unresolved.65
A clinical spectrum of disease exists, with some infants exhibiting relatively mild and limited disease involving several days of constipation, poor feeding, and lethargy, and other infants developing acute tetraparesis and respiratory failure.65 In classic cases, constipation is often the initial symptom, followed by lethargy, poor feeding, and weak cry. Examination reveals hypotonia with head lag, ptosis, reduced facial expression, and reduced gag, suck, and swallow reflexes. Deep tendon reflexes are reduced or absent. Extraocular movements are often paretic, and pupils may be large and poorly reactive. In one series, more than half of the patients were intubated and mechanically ventilated, usually following loss of protective upper airway reflexes.66 Although the course is variable, most hospitalized infants reach maximal paralysis at approximately 1 to 2 weeks after hospitalization and begin to improve after 1 to 3 weeks.65 In California between 1976 and 1991, the average length of hospitalization was 4.9 weeks. A longer length of stay was documented for type A cases (5.7 weeks) compared with type B cases (3.6 weeks), suggesting that type A intoxication causes more severe disease.65 The case-fatality rate is less than 1% in hospitalized patients in the United States.56
Adult Intestinal Botulism
Children and adults also may be susceptible to intestinal colonization and in vivo toxin production by C. botulinum, C. baratii, or C. butyricum when the gastric barrier is compromised and the intestinal flora are altered.8,15,67,68 Previously classified by the Centers for Disease Control and Prevention (CDC) as “botulism of undetermined origin,” adult intestinal botulism has occurred in the setting of intestinal surgery, gastric achlorhydria, broad-spectrum antibiotic treatment, and inflammatory bowel disease.69–71 Although adult intestinal botulism is uncommon (10 cases reported between 1986 and 1996),71 it is probably underdiagnosed.
Inhalational Botulism
Inhalational botulism does not occur in nature but is the result of an attempt to use the toxin in aerosolized form as a bioweapon.10 The three documented human cases were reported from Germany in 1962, when botulinum toxin type A became accidentally reaerosolized during disposal of laboratory animals.9 These patients initially developed dysphagia on day 3 after exposure and exhibited tonic pupils, paretic eye movements, dysarthria, and diffuse weakness by day 4. In animal experiments, monkeys became symptomatic 12 to 18 hours after exposure to aerosolized toxin, with descending paralysis and death in some animals.72 Aerosolized botulinum toxin was released by the Japanese religious cult, Aum Shinrikyo, on several occasions in the 1990s in Japan, although the attacks were not known to have produced human illness.10 By the time of the 1991 Persian Gulf War, the state of Iraq had produced large quantities of concentrated botulinum toxin which were loaded onto weapons for military use but never deployed.73
Release of aerosolized toxin has the potential to produce a botulism outbreak. The features of such an outbreak that might suggest a deliberate release of toxin10 include: numerous cases within an outbreak (the mean number of cases in foodborne outbreaks has averaged 2.5 for many years)12; toxin types within an outbreak that rarely cause natural disease (type C, D, F, G, or E not related to marine sources); outbreaks with a common geographic factor without a common dietary exposure; and multiple simultaneous outbreaks.74
Iatrogenic or Inadvertent Botulism
The therapeutic use of botulinum toxin for dystonia, spasticity, hyperhidrosis, sialorrhea, and other conditions occasionally has resulted in inadvertent paresis of nearby noninjected muscles, such as dysphagia in neck muscle injections for cervical dystonia75 and jaw dislocation after parotid injections for sialorrhea in amyotrophic lateral sclerosis.76 Although there are rare reports of paretic muscles distant to the site of injection,77,78 it has been estimated that healthy patients would require a 10-fold toxin overdosing to develop systemic symptoms.79 Nevertheless, single-fiber electromyography studies showed abnormal neuromuscular transmission in muscles distant to botulinum toxin injections.80–82 Patients with underlying neuromuscular disorders seem to be predisposed to developing generalized weakness after therapeutic intramuscular botulinum toxin injections.83–85 Systemic autonomic dysfunction also has been noted after therapeutic injections of type B toxin.86
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree