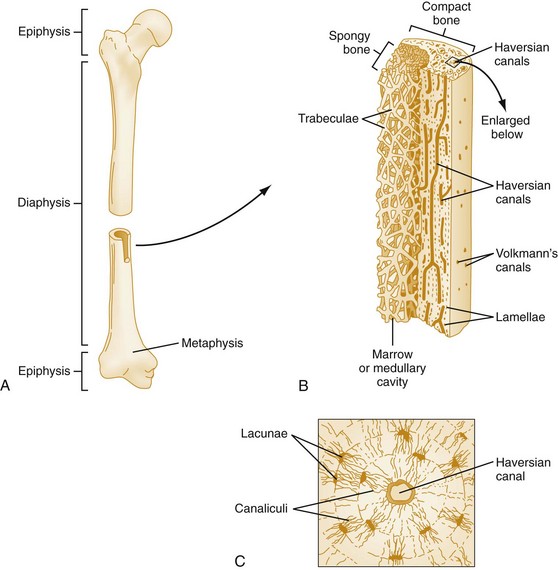
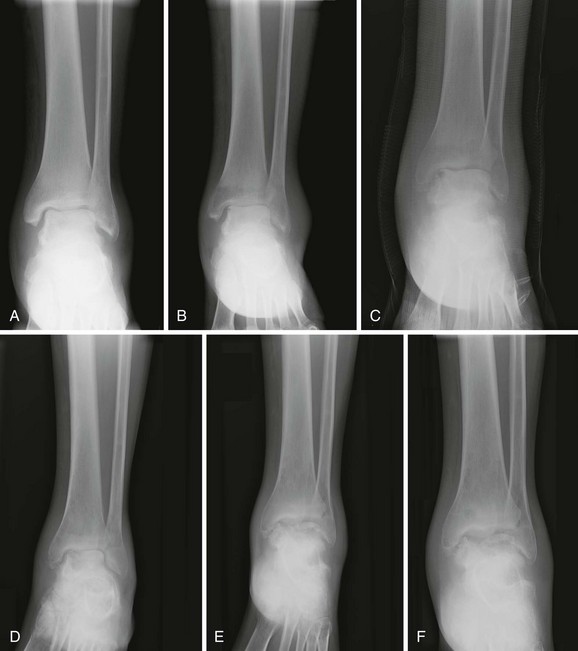
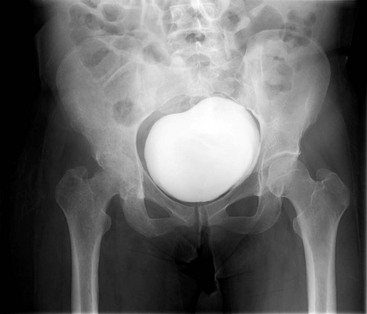
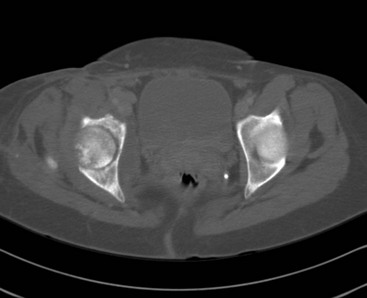
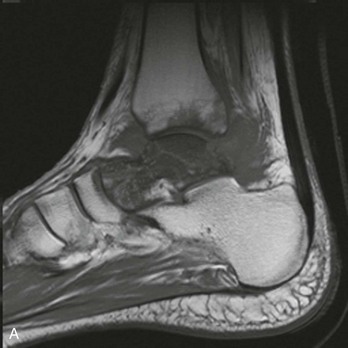
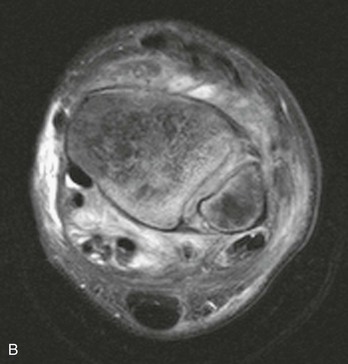
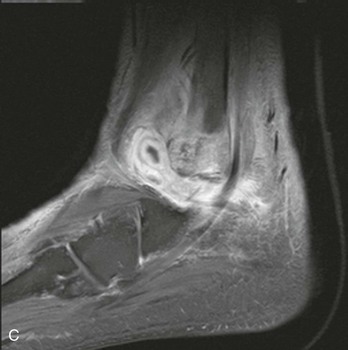
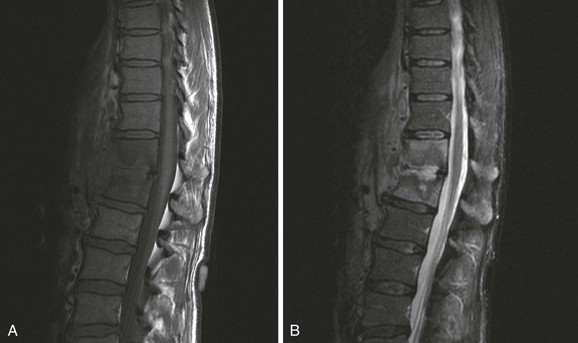
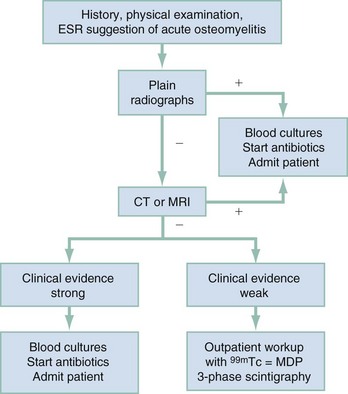
Chapter 136 Historically, bone and joint infections have been described in grim terms. Aids to Surgery, written in 1919, notes that “acute infective osteomyelitis … is a very fatal disease.” With septic arthritis, “the patient becomes exhausted from toxaemia or pyemia,” and “ankylosis is the usual most favourable termination.”1 Advances in diagnostic methods, antibiotic therapy, and surgical techniques have resulted in better patient outcomes. In the case of osteomyelitis, treatment with antibiotics and limb salvage protocols is successful in more than 90% of patients with chronic osteomyelitis despite the increase in the number of patients with multiple comorbidities that affect wound healing. However, new challenges are being unveiled. The types of infections that are encountered today are evolving. Host immunity is decreased in many populations, leading to the complexity of the management of bone and joint infections that has never before been encountered. Emergency physicians must consider many subsets of patients who are at increased risk of infection, including injection drug users, patients with acquired immunodeficiency syndrome (AIDS), postsurgical patients, and patients with iatrogenic immune suppression. The emphasis of modern management of bone and joint infections has shifted from prevention of sepsis and death to prompt diagnosis, initiation of treatment, and avoidance of the complications and morbidity associated with chronic bone or joint infections. The overall occurrence of bone and joint infections appears to have remained constant during the past three decades.2 The incidence of bone infections in hospitalized patients is approximately 1%. In the United States, the incidence of osteomyelitis in children younger than 13 years is 1 in 5000, whereas the incidence of septic arthritis ranges from 5.5 to 12 per 100,000 individuals.2 Globally, epidemiologic data for community-acquired bone and joint infections in adults vary significantly, with an overall higher incidence in patients of a lower socioeconomic class in developing countries. However, in the United States, there is no correlation between socioeconomic factors or race and the incidence of bone and joint infections. Both bone and joint infections show a bimodal age distribution, occurring most commonly in people younger than 20 years or older than 50 years. In children, bone and joint infections usually occur in previously healthy individuals, and boys have a slightly increased susceptibility to bone infections.3 In adults, risk factors can usually be identified in patients who present with either bone or joint infection. Septic arthritis is defined as an infection of a joint by bacterial or fungal organisms. Bacterial arthritis is sometimes called pyogenic or suppurative arthritis. Reactive arthritis is more common than bacterial arthritis. It is a sterile, secondary inflammation of a joint with no identifiable infecting microorganisms within the synovial fluid. Commonly, reactive arthritis occurs after a systemic infection with a virus,3 but it can also develop after group A streptococcal infection. The gross structure of long bones can be divided into several sections. The diaphysis is the shaft of the bone and contains the compact cortical bone with an overlying periosteum, the medullary canal containing marrow, and a small amount of trabecular bone. The metaphysis is the junctional region between the epiphysis and the diaphysis. The metaphysis contains abundant trabecular bone, but the cortical bone thins here relative to the diaphysis. Finally, the epiphysis is the area at either end of a long bone. The epiphysis is made up of abundant trabecular bone and a thin shell of cortical bone (Fig. 136-1). In general, in the skeletally mature individual, the epiphysis is involved in articulation and is not covered by a periosteum but instead has a thin layer of articulating cartilage. After the first year of life, there is no longer a vascular connection between the metaphysis and epiphysis. The metaphyseal arteries end in loops that abut the growth plate. The epiphyseal growth plate is avascular and inhibits the spread of infection to the epiphysis and joint. Instead of spreading to the joint space, the infection usually spreads laterally through Volkman’s canals, breaks through the cortex, and lifts the loose periosteum to form a subperiosteal abscess.4 Bacteria congregate in a highly structured community, the biofilm, which plays an important role in the pathogenesis of septic arthritis and osteomyelitis.5 In the biofilm community, bacteria communicate with each other through cell-cell signals and adhere to both inert and living surfaces. Bacterial cells can be released from the biofilm and revert to the individual planktonic state. The biofilm community is more resistant than its planktonic counterparts to the host immune response and antibiotics. First, there is a protective layer on the biofilm, the biofilm matrix, that is difficult to penetrate. Second, the biofilm secretes catalase, which protects the community from hydrogen peroxide, which is used by neutrophils to kill bacteria. Finally, the community is protected by its metabolic variability. Within the biofilm, the bacteria are at varying stages of metabolism; some are active, some are slow growing, and some are dormant. Antibiotics target metabolically active bacteria, such as those in the planktonic state, but bacteria in other stages in the biofilm community may be more resistant to the effects of antibiotics. When bacterial infections form biofilms in bone, in joints, or on artificial implanted devices, fewer free bacteria are available to be aspirated. This helps explain why Gram’s stains of aspirated synovial fluid in a suspected septic joint are often negative because Gram’s stain will identify only planktonic bacteria. It also helps explain why a definitive diagnosis is made only by a culture of the synovial fluid aspirate or synovial tissue. Biofilm formation is one of the reasons that optimal treatment of a septic joint, especially of prosthetic joints, involves complete surgical débridement.5 Infections from direct implantation of bacteria are caused by deep puncture wounds and tend to occur in the hands and feet. Human and animal bites are another cause of infections that can result in osteomyelitis and septic arthritis. Although cats account for only 10% of animal bites, significant infection results from 20 to 50% of cat bites versus only 5% of dog bites because of the morphology of feline teeth. Most human bite injuries are related to fistfights and contamination of the metacarpophalangeal joints and metacarpals, with infection due to oral flora. Direct implantation of pathogens is also common with open fractures and can also occur during surgical instrumentation. Artificial joints and other implanted surgical materials can serve as sites for colonization of bacteria and formation of biofilms.6 Septic arthritis is usually a consequence of hematogenous spread unless there is direct injection of bacteria into the joint. The lack of a basement membrane makes the highly vascular synovium vulnerable to bacterial seeding. Infection occurs first in the synovium, spreads into joint fluid, and finally affects the articular cartilage. Bacterial enzymes and toxins directly damage cartilage. The synovial membrane responds to infection by increasing synovial fluid production, resulting in a large joint effusion. Septic arthritis is a closed space infection; increasing pressure in the joint contributes to a decreased rate of exchange of solutes across the synovial lining and synovial blood flow can be reduced by the increased pressure, resulting in ischemic damage to cartilage. Even a small bacterial load in the joint space elicits a profound and persistent inflammatory and immune response. Bacteria can be cleared from the joint, resulting in a sterile-appearing inflammatory response. In animal models, the injection of isolated bacterial DNA into joints can produce a marked inflammatory arthritis.7 In response to infection, synovial cells and polymorphonuclear leukocytes release lysosomal enzymes into joint fluid. These enzymes contain collagenase and elastase, both of which can degrade cartilage. Cytokines also seem to play a key role in the release of metalloproteinases and other harmful enzymes. The most pathologic aspect of septic arthritis is the destruction of articular cartilage, which creates a painful joint with limited motion. Once it is destroyed, hyaline (articular) cartilage is not replaced. Other structures that are enclosed within or adjacent to the synovium, such as bursae, tendons, and bone, may become damaged in septic arthritis.7 The pathogenic organisms in osteomyelitis are numerous, and Gram-positive organisms are responsible for most types of osteomyelitis. Even though Gram-negative organisms account for 43% of cases of community-acquired bacteremia, they result in only about 10% of septic arthritis cases. Trauma predisposes patients to osteomyelitis by environmental pathogens. Patients who are wounded or sustain open fractures in fresh water are susceptible to infections with the Gram-negative bacillus Aeromonas hydrophila. People who are bitten by animals, particularly dogs and cats, are at risk for development of osteomyelitis from Pasteurella multocida.6 Osteomyelitis caused from human bites is most common in the hand and involves human oral flora, such as Streptococcus anginosus, Fusobacterium nucleatum, and Eikenella species. In the population of injection drug users, S. aureus is the most likely cause of infection, followed by Pseudomonas species. Pseudomonas aeruginosa is also an important cause of osteomyelitis in puncture wounds, in postsurgical wounds, and in patients with sickle cell anemia. Certain underlying disease states predispose a patient to acquire bone and joint infections. These conditions include diabetes mellitus, sickle cell disease, AIDS, alcoholism and injection drug use, chronic corticosteroid use, preexisting joint disease (especially rheumatoid arthritis), and other immunosuppressed states. Another subset of patients who are susceptible to bone and joint infections are postsurgical patients, especially those who have implanted prosthetic devices (Table 136-1). Table 136-1 Microbiology of Bacterial Septic Arthritis as Related to Age of the Patient †With widespread immunization. Reprinted with permission from Esterhai JL Jr, Rao N: The epidemiology of musculoskeletal infections. In Cierny G 3rd, McLaren AC, Wongworawat MD (eds): Orthopaedic Knowledge Update: Musculoskeletal Infection. Rosemont, Ill, American Academy of Orthopaedic Surgeons, 2009, 8. Modified from Liu NY, Giansiracusa DF: Septic arthritis. In Gorbach SL, Bartlett JG, Blacklow NR (eds): Infectious Diseases, ed 2. Philadelphia, WB Saunders, 1998, 1345. Although most bone and joint infections are bacterial, viruses, fungi, and parasites may be the responsible pathogens. The microbiology of osteomyelitis and septic arthritis is a function of several host and environmental factors. As has been described, age is an important variable in determining the type of bacteria that cause bone and joint infections. A patient’s living environment also has some role in determining the incidence of bone and joint infections. For example, people living in crowded conditions where tuberculosis is prevalent are at increased risk for tubercular bone and joint infections. Elder patients in hospitals and institutions may be more susceptible to infections with Gram-negative bacteria. A summary of the organisms that cause osteomyelitis and septic arthritis is given in Table 136-2. The following points deserve special mention: Table 136-2 Microbiology and Initial (Empirical) Antibiotic Treatment of Bone and Joint Infection *Concurrent treatment of Chlamydia trachomatis infection should be given to patients with suspected N. gonorrhoeae septic arthritis. • In all age groups except neonates, S. aureus is the leading cause of osteomyelitis. It also accounts for more cases of septic arthritis than any other bacterium. In neonates, group B streptococci, Escherichia coli and other Gram-negative coliforms, and Staphylococcus epidermidis are the most common pathogens responsible for bone and joint infections.8 • Since the introduction of the vaccine, Haemophilus influenzae type b, once a common cause of septic arthritis and osteomyelitis in children younger than 2 years, has essentially disappeared as a pathogen among vaccinated children.9 Another Gram-negative coccobacillus within the Neisseriaceae family, Kingella kingae, in being encountered with increasing frequency. K. kingae can be part of the normal flora of the nasopharynx; like H. influenzae, it can be spread hematogenously to bones and joints. It is a fastidious organism and may be mistaken for Haemophilus or Neisseria species. • P. aeruginosa has been reported as a cause of cervical spine osteomyelitis in injection drug users and lumbar spine osteomyelitis in patients with urinary catheters in place for long times. Pseudomonas colonizes the rubber and plastic inserts in footwear and is therefore seen in soft tissue infections and osteomyelitis of the foot after a puncture wound. • In elders and in patients with diabetes, Gram-negative bacteria account for a higher percentage of cases of bone and joint infections. • Methicillin-resistant S. aureus (MRSA), methicillin-resistant S. epidermidis, and vancomycin-resistant enterococci have emerged as a significant microbiologic problem in the past decade. Multiresistant enterococci pose the greatest potential danger in that no currently available antibiotic regimen is reliably bactericidal against such organisms. A typical case of hematogenous osteomyelitis or septic arthritis is caused by a single strain of bacterium, although polymicrobial infection occurs 36 to 50% of the time and is more likely to occur in diabetic foot osteomyelitis, post-traumatic osteomyelitis, and chronic septic arthritis or osteomyelitis. Anaerobic bacteria can complicate polymicrobial infection and may be present in bone and joint infections more often than is commonly recognized because standard culture techniques may be inadequate for isolation of anaerobic bacteria.10 This is especially important in chronic osteomyelitis, in which anaerobic bacteria may be present in up to 40% of cases. Mycobacterium tuberculosis may infect bones and joints, most commonly in the axial skeleton. The two most common forms of skeletal infection are vertebral osteomyelitis (Pott’s disease) and tubercular arthritis. The spine is affected in half of cases of tubercular skeletal infection. The arthritis of tuberculosis is a chronic, low-grade inflammatory process that resembles rheumatoid arthritis more than acute septic arthritis.11 The prevalence of fungal bone and joint infections is increasing because of the rise in the number of patients with risk factors. Host factors that predispose to fungal infections include immobility, mucositis, use of antibiotics, radiation therapy, immunosuppressive agents, admission to an intensive care unit, malnutrition, and hematopoietic stem cell transplantation.12 Fungal infection is also a complication of catheter-related fungemia, the use of injection drugs contaminated by Candida species, and prolonged neutropenia. Candida osteomyelitis occurs through hematogenous spread or as a postoperative wound infection. Fungal bone infection is indolent and may go through periods of activity and remission.12 Aspergillus has been reported to cause osteomyelitis in vertebrae, hip prostheses, and ribs. In adults, the infection is hematogenous. In children, Aspergillus osteomyelitis is most common in those with prior granulomatous disease and spreads from a primary pulmonary infection. Blastomyces and Cryptococcus are two other fungi that may become disseminated and infect the skeleton. Treatment of these infections is difficult, especially because of emerging pathogens that are resistant to common antifungal agents, and therapy needs to be individualized against the specific organism. The symptoms and signs of osteomyelitis in adults are predictable, although not always present. Patients with osteomyelitis often present with fever and rigors and may appear toxic. Fever appears to be present more commonly in children. Systemic complaints of headache, fatigue, malaise, and anorexia are often reported.13 However, these findings are inconsistently present and are less likely with chronic osteomyelitis. In children with lower extremity osteomyelitis, a sudden limp or inability to bear weight, localized warmth, swelling, and erythema may be reported. A careful review of the patient’s past medical history is especially important to identify risk factors that may predispose to bone infection. Laboratory Data and Diagnostic Imaging Laboratory data are not specific and can only suggest the diagnosis of osteomyelitis. In acute osteomyelitis, the white blood cell (WBC) count is often although not always elevated; typical values range from normal to 15,000/mm3. The WBC count is often normal in chronic osteomyelitis. The erythrocyte sedimentation rate (ESR), a nonspecific measure of inflammation, is more helpful than the WBC count. The ESR represents the rate at which red blood cells fall when anticoagulated blood is placed in an upright tube. When an inflammatory process is present, the high proportion of fibrinogen in the blood causes the red blood cells to form stacks, called rouleaux, which settle faster. This is a sensitive marker for bone infection, and many series report elevated ESRs in patients who have confirmed osteomyelitis. In children, an elevated ESR or C-reactive protein (CRP) level is seen in all cases of osteoarticular infection, and the sensitivity of use of both the ESR and the CRP value is 98%.14 In the evaluation of a diabetic foot infection, an ESR greater than 70 mm/hr predicts the presence of an underlying bone infection.15 An elevated ESR in the presence of appropriate physical findings should lead one to suspect osteomyelitis, but a normal or slightly elevated ESR does not eliminate the diagnosis. Other inflammatory conditions, such as cellulitis, can cause an elevated ESR, although the degree of elevation of the ESR is often higher with osteomyelitis. The ESR and CRP blood tests have a high positive predictive value, and elevated values should increase suspicion for this diagnosis. However, normal values of these nonspecific inflammatory markers cannot be used to rule out the diagnosis of osteomyelitis. The CRP may be a better early indicator of disease, but the ESR is most valuable in following response to treatment.16 Typically, the ESR falls steadily as osteomyelitis resolves and increases should it recur. Because conventional radiography is readily available, relatively inexpensive, and useful in differentiation of infection from trauma and tumors, it remains the initial imaging test of choice for suspected osteomyelitis. In addition, plain radiography is often a helpful adjunct to secondary imaging studies. Unfortunately, radiographic evidence of osteomyelitis lags behind the clinical picture, and less than one third of patients have abnormalities on plain radiographs in the first 7 to 10 days after the onset of symptoms. The characteristic early findings on the plain radiograph in osteomyelitis are lucent lytic areas of cortical bone destruction (Fig. 136-2). However, lucency is not detected on radiographs until approximately 50% of bone mineral is lost, and this often takes up to 2 weeks from the onset of infection. Although these findings are often difficult to identify on plain radiographs, soft tissue edema, deep soft tissue swelling, distorted fascial planes, and altered fat interfaces may be present within 3 to 5 days from the onset of infection and can serve as a clue to osteomyelitis in the underlying bone. Periosteal reaction is another early sign. It may appear on radiographs as hypertrophy or elevation of the periosteum creating an involucrum (Fig. 136-3). Because of the thinner periosteum, these early periosteal changes are seen in radiographs in children more commonly than in adults. In advanced disease, the lytic lesions are surrounded by dense, sclerotic bone, and sequestra may be noted. By 28 days from the onset of osteomyelitis, 90% of the plain radiographs are abnormal.17 Radionuclide skeletal scintigraphy (bone scanning) is more sensitive than plain radiography in the early diagnosis of osteomyelitis and is especially useful in the presence of prosthetics or other hardware. Radionuclide scans can detect osteomyelitis within 48 to 72 hours after the onset of infection. A radioactive tracer is injected into the bloodstream and given time to bind or to accumulate in body tissues, after which a camera is used to sense released radioactivity. An image is created that is evaluated for increase or decrease in expected uptake of the radionuclide. In the past decade there has been a movement away from skeletal scintigraphy in diagnosis of osteomyelitis. There is a significant radiation burden associated with scintigraphy, and recent recommendations are that children thought to have acute osteomyelitis should have magnetic resonance imaging (MRI) rather than a bone scan.18 Computed tomography (CT) may be useful in the diagnosis of osteomyelitis. The bony cortex is particularly well seen on CT, and involucrum and sequestrum formation is easily identified. Non-long bones like the sternum, vertebrae, pelvic bones, and calcaneus are far better imaged with a CT scan than with plain radiographs. CT is most commonly used to detect and to define areas of possible infection in bones with complex anatomy that is difficult to visualize on plain radiographs and bone scans. Osteomyelitis appears as rarefaction, or lucent areas, on the CT scan images (Fig. 136-4). Gas may be seen in bony abscess cavities. The limitation of CT for early diagnosis of osteomyelitis is the same as that for plain radiography in that the disease must be present for more than a week for changes to be apparent. An important role of a CT scan in osteomyelitis is to help localize bone lesions that have been found with other imaging modalities. The CT scan can guide the surgeon in débridement and resection of infected bone and in choosing a site for diagnostic biopsy.17 The use of bone scans and CT for detection of osteomyelitis in the emergency department (ED) is decreasing as the availability and image quality of MRI improves while its cost decreases. The anatomic resolution of MRI is far superior to that of bone scans and plain radiographs. MRI findings are often evident before an abnormality is detected by skeletal scintigraphy, plain radiography, or CT because of the earlier detection of bone marrow involvement. MRI can reveal edema and the destruction of the medulla as well as any periosteal reaction, cortical destruction, articular damage, and soft tissue involvement, even when conventional radiographs are still normal. Whereas the presence of ferromagnetic material is a contraindication to MRI, most materials used in orthopedic surgery, such as titanium and chrome cobalt, do not interfere with this imaging modality. One drawback to MRI is the presence of metal in bone, especially prosthetic joints. Metal may cause distortion of the signal in the area adjacent to a joint prosthesis, but this does not exclude MRI in this group of patients.17 MRI uses a combination of spin echo T1-weighted and T2-weighted images, short tau inversion recovery (STIR) images, and fat-suppressed T2-weighted images. Osteomyelitis produces a diminished intensity of the normal marrow signal on T1-weighted images and a normal or increased signal on T2-weighted images18 (Fig. 136-5). Administration of gadolinium as a contrast agent enhances the interface between normal and abnormal marrow and helps distinguish devitalized from normally perfused bone. Gadolinium becomes localized in areas of increased vascularity and blood flow and also helps distinguish soft tissue infections such as abscesses and cellulitis from osteomyelitis. Whereas contrast agents increase reader confidence in the diagnosis of osteomyelitis, they do not increase the sensitivity or specificity of the diagnosis of osteomyelitis.17 Soft tissue swelling and edema are best detected with fat-suppressed images, in which the resolution is much greater with MRI than with plain radiographs or CT scan. MRI also allows better visualization of subtle bone cortical changes, periosteal reaction, soft tissue edema, abscesses, and sinus tracks. When it is done with STIR images, MRI has a 100% negative predictive value, and osteomyelitis can be essentially excluded in the presence of a normal MRI study.17 STIR images have a very low signal from fat, like fat-suppressed images, but still obtain a strong signal from water. The sensitivity of MRI is reported to be between 88 and 100%, with a specificity of 75 to 100%.17 MRI with gadolinium contrast enhancement has been demonstrated to have higher sensitivity and specificity than radionuclide bone scans in the diagnosis of vertebral osteomyelitis17,19,20 (Fig. 136-6). Particularly in cases of hematogenous osteomyelitis, cultures of blood, urine, cerebrospinal fluid, and pus from other sites of infection can help uncover the infecting bacteria. Blood cultures in patients with acute untreated osteomyelitis are positive for the offending bacteria approximately 50% of the time.21 In children with hematogenous osteomyelitis, it is not unusual to identify the infecting organism in other body fluid cultures in addition to blood cultures. In chronic osteomyelitis, blood cultures are almost always negative.18 The likelihood of establishing a bacteriologic diagnosis in acute osteomyelitis is 80 to 90%, but in some cases even culture of resected bone yields no organism. Possible reasons for this are poor culture techniques and inadequate preparation of recovered tissue for culture, previous antibiotic treatment, and culture specimens from necrotic ischemic regions that may be devoid of bacteria.18 The emergency physician’s diagnostic approach in suspected osteomyelitis has become simpler since MRI has largely replaced the use of radionuclide scintigraphy. The algorithm in Figure 136-7 provides a simplified approach to the diagnostic strategy in a patient with suspected osteomyelitis. A few key points should be considered with use of this algorithm:
Bone and Joint Infections
Perspective
Principles of Disease
Etiology and Microbiology of Bone and Joint Infections
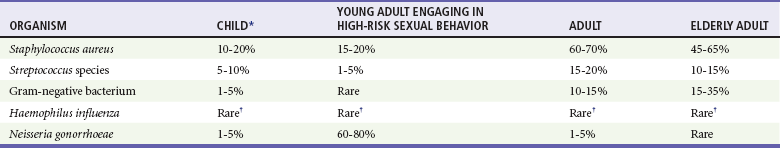
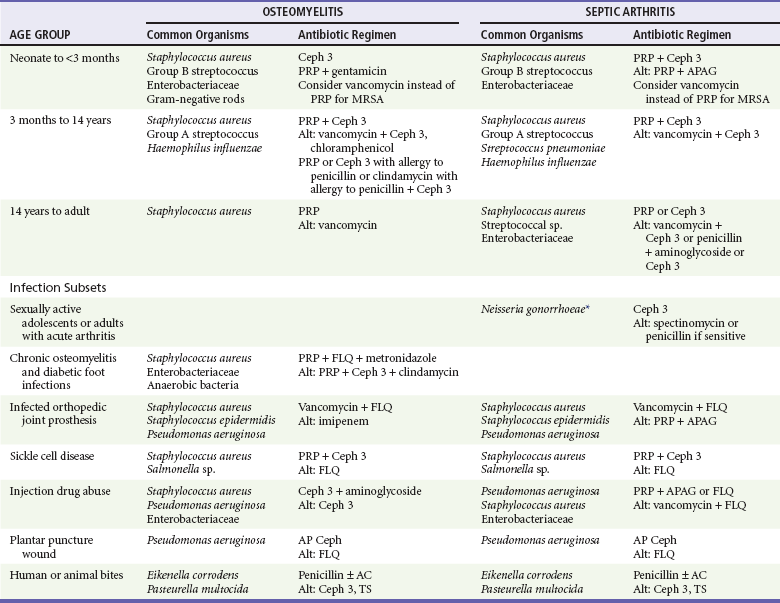
Osteomyelitis
History and Physical Examination
Diagnostic Strategies
Microbiologic Diagnosis
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bone and Joint Infections
Only gold members can continue reading. Log In or Register to continue