Fig. 78.1
Arrows mark site of active extravasation following splenic trauma
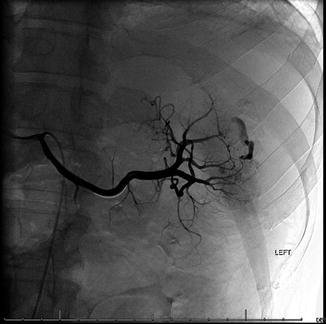
Fig. 78.2
Splenic bleeding site confirmed by angiography
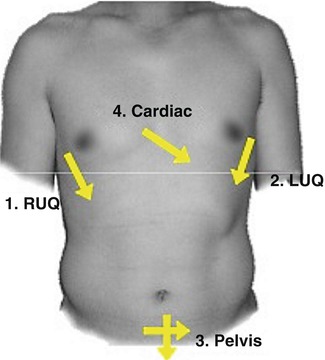
Fig. 78.3
FAST – Location of probe placement for the trauma examination (u.surgery. (2009). Focused Abdominal Sonography for Trauma [PowerPoint slides]. Retrieved from http://www.slideshare.net/u.surgery/focused-abdominal-sonography-for-trauma)
Principles of Management
Unstable Versus Stable Blunt Abdominal Trauma
The initial management of blunt intra-abdominal injuries depends crucially on whether the patient is hemodynamically stable or unstable. Trauma patients who are unstable are bleeding until proven otherwise, and prompt evaluation is indicated to determine the source of bleeding. There are 5 areas into which a trauma patient can bleed to death – the chest, the abdomen, the pelvis and retroperitoneum, the thigh and externally [1].
The location of bleeding can be determined quickly with minimal testing in the trauma bay. A chest x-ray and pelvis film will determine if a patient has a massive hemothorax or an open pelvic fracture, respectively. The FAST exam rapidly evaluates 4 areas: the pericardium, the area between liver and right kidney, the area between spleen and left kidney, and the suprapubic area, with any free fluid presumed to represent hemorrhage [1]. Alternatively, a diagnostic peritoneal aspiration (DPA) or lavage (DPL) can be used to determine if there is fluid or blood within the peritoneal cavity.
Patients with blunt injury who are hemodynamically unstable with evidence of intraperitoneal hemorrhage on FAST or DPL should be taken to the operating room for an immediate laparotomy [5–9]. Patients who are hemodynamically stable can proceed with further 3D imaging and nonoperative management. The current management of blunt hepatic and splenic injury is selective nonoperative management (NOM) with operative management in those patients who present with hemodynamic instability or have ongoing evidence of bleeding [9–12].
Balanced Resuscitation
A tenet of trauma resuscitation is ensuring that patients have appropriate intravenous access [1]. Most patients can be managed with two large-bore (14–16 g) peripheral intravenous catheters. The type and amount of IVF that is optimal for trauma patients is constantly debated. Crystalloids are associated with improved survival in trauma patients compared to colloids [13]. Lactated Ringer’s is preferred to Normal Saline because it is associated with less metabolic acidosis in the setting of massive hemorrhagic shock in animal models [14].
The Inflammation and Host Response to Injury Project defined a systolic blood pressure less than 90 mmHg and/or a heart rate greater than 130 beats per minute as indicative of shock in a traumatically injured patient [15]. ATLS guidelines also recommend the initial administration of 1–2 l of isotonic crystalloid in the resuscitation of a trauma patient [1]. For a patient that requires further resuscitation, the administration of blood products is recommended, as excessive crystalloid resuscitation has been associated with increased morbidity and length of stay in blunt trauma patients [16]. Two recent trials investigating the timing and ratio of blood product administration have shown improved mortality with the early administration of plasma [17] and better hemostasis with fewer deaths from exsanguination without adverse effects with the administration of blood, plasma and platelets in a 1:1:1 ratio [18].
Prompt hemorrhage control should be the main goal of hemorrhagic shock management, and can be accomplished through the use of external hemorrhage control, Interventional Radiology for angioembolization or a surgical procedure.
Imaging and Diagnosis
Solid organ injury after blunt abdominal trauma in stable patients is best visualized by CT scan abdomen and pelvis with IV contrast [5–8]. The severity of liver and spleen injuries can be classified according to the American Association for the Surgery of Trauma organ grading scales (Tables 78.1 and 78.2) [19]. Blunt hollow viscus injury is uncommon but should be suspected in patients with extraluminal air on 3-D imaging, frank succus or particulate material on peritoneal lavage or evolving peritonitis on serial examination.
Table 78.1
Spleen injury scale
Grade | Injury type | Description of injury | AIS-90 |
|---|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area | 2 |
Laceration | Capsular tear, <1 cm parenchymal depth | 2 | |
II | Hematoma | Subcapsular, 10–50 % surface area intraparenchymal, <5 cm in diameter | 2 |
Laceration | Capsular tear, 1–3 cm parenchymal depth that does not involve a trabecular vessel | 3 | |
III | Hematoma | Subcapsular, >50 % surface area of expanding; ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >5 cm or expanding | 3 |
Laceration | >3 cm parenchymal depth or involving trabecular vessels | 3 | |
IV | Laceration | Laceration involving segmental or hilar vessels producing major devascularization (>25 % of spleen) | 4 |
V | Laceration | Completely shattered spleen | 5 |
Vascular | Hilar vascular injury which devascularizes spleen | 5 |
Table 78.2
Liver injury scale
Grade | Injury type | Description of injury | AIS-90 |
|---|---|---|---|
I | Hematoma | Subcapsular, <10 % surface area | 2 |
Laceration | Capsular tear, <1 cm parenchymal depth | 2 | |
II | Hematoma | Subcapsular, 10–50 % surface area intraparenchymal <10 cm in diameter | 2 |
Laceration | Capsular tear 1–3 parenchymal depth, <10 cm in length | 2 | |
III | Hematoma | Subcapsular, >50 % surface area of ruptured subcapsular or parenchymal hematoma; intraparenchymal hematoma >10 cm or expanding | 3 |
Laceration | >3 cm parenchymal depth | 3 | |
IV | Laceration | Parenchymal disruption involving 25–75 % of hepatic lobe or 1–3 Couinaud’s segments | 4 |
V | Laceration | Parenchymal disruption involving >75 % of hepatic lobe or >3 Couinaud’s segments within a single lobe | 5 |
Vascular | Juxtahepatic venous injuries; ie, retrohepatic vena cava/central major hepatic veins | 5 | |
VI | Vascular | Hepatic avulsion | 6 |
Nonoperative Management (NOM) of Blunt Solid Organ Injury
Patients who are hemodynamically stable without peritonitis and are found to have a blunt spleen or liver injury can undergo NOM [5–8, 10, 20]. NOM involves a period of in-hospital observation, serial abdominal examinations, serial hematocrit measurements and possibly a period of bedrest [5, 6]. NOM should be undertaken in an environment and institution where patients can be appropriately monitored, undergo serial abdominal exams and the capability to provide operative intervention is readily available. Blunt kidney injuries are, in general, also treated successfully with NOM.
Angioembolization for Blunt Solid Organ Injury
Angioembolization should be considered as an adjunct to nonoperative management of blunt splenic injury in patients with a grade 3 or higher injury, a contrast blush on CT scan, moderate hemoperitoneum on CT scan and evidence of ongoing bleeding [5, 6]. Having an institutional protocol for angioembolization has led to decreased LOS and decreased use of hospital resources [21]. The implementation of protocols for angioembolization in patients who are high risk for failure of NOM (contrast blush and grades 3–5) are associated with increased success of NOM [22, 23]. For blunt hepatic injuries, angioembolization should be considered for stable patients with contrast extravasation on CT. Early embolization in blunt hepatic injury is associated with decreased transfusion requirements and decreased need for hepatic operative intervention [24, 25]. Angioembolization can also be used as an adjunct to operative management [26–28].
Post-splenectomy Vaccinations
An initial report by King and Schumacker in 1951 documented severe infection after splenectomy in infants [29]. Since then, overwhelming post-splenectomy infection (OPSI) and mortality from it has been documented and recognized in asplenic patients from a variety of different mechanisms, including patients who have undergone a splenectomy due to trauma [30]. The CDC recommends ensuring a complete vaccination panel after splenectomy: 13-valent and 1, 2 or 3 doses of 23-valent pneumococcal vaccine depending on previous vaccination, two doses of quadrivalent meningococcal vaccination followed by a dose every 5 years, Haemophilus Influenza type B vaccination and evaluation for influenza, Td/Tdap [tetanus, diphtheria, pertussis), varicella, human papillomavirus, zoster and measles, mumps, rubella vaccines [31]. Shatz and colleagues found that administration of vaccinations at 2 weeks post-splenectomy were associated with the best antibody response compared to vaccination at 1, 7, or 28 days [32].
Evidence Contour
Who Should Be Managed Nonoperatively?
Previously, age greater than 55, neurologic status, high grade of injury and associated injuries were considered contraindications to NOM of blunt splenic injury. Subsequent studies have shown that NOM is feasible and safe in these populations, although patients greater than 55 years old have a higher mortality rate with blunt splenic injury despite the choice of management strategy [33, 34]. These patients had a higher mortality with failure of NOM than the younger cohort [35]. Head injury or altered mental status is also not a contraindication to NOM of either hepatic or splenic injuries [36]. A review from 2013 cautioned clinicians to be aware of factors in the literature which are associated with increased failure of NOM: age greater than 40 years old, ISS of 25 or greater, and a AAST splenic injury grade 3 or higher [37]. Most studies agree that increasing grade of injury and an increased ISS are associated with an increased rate of failed NOM, but we are still able to achieve high levels of NOM success in these patients [11, 38, 39]. Patients with multiple injuries, including multiple solid organ injuries, can be managed nonoperatively, although they do have a higher failure rate [40]. For blunt hepatic injuries, intraperitoneal contrast and hemoperitoneum in multiple quadrants are predictive of the need for operative intervention, even in hemodynamically stable patients [41].
How Should Nonoperative Management Be Accomplished?
There are no guidelines published to outline the timing and frequency of hematocrit measurements, serial abdominal examinations, length of monitoring and duration of bed rest, if at all. A retrospective cohort study of blunt solid organ injury and the timing of mobilization did not demonstrate an increase in delayed hemorrhage based on early mobilization, and led the authors to conclude that bed rest should not be a part of NOM protocols for blunt solid organ injury [42]. Centers with established protocols for NOM have decreased LOS and a low rate of NOM failure. A protocol with clear inclusion and exclusion criteria for NOM along with an outline for the frequency and duration of serial abdominal examinations, hematocrit draws and length of bed rest has led to a decrease in hospital and ICU LOS and an increase of NOM success without an increase in mortality [43–45].
Is Follow-Up Imaging Necessary?
For blunt splenic injury managed initially without angioembolization, the need for or timing of follow up imaging is not clearly documented in the literature. A Delphi consensus statement regarding blunt splenic injury found a fifty-fifty split between experts regarding the need for repeat imaging during the initial hospital admission [9]. Shapiro and colleagues found that, among their trauma population, in the absence of clinical signs and symptoms of bleeding, a repeat CT scan did not change management [46]. However, subsequent studies have suggested that repeat imaging allows for the identification and subsequent angioembolization of splenic artery pseudoaneurysm (SPA) or arterial extravasation (AE) and reduces failure of NOM. Weinberg and colleagues described a protocol of repeat CT imaging at 24–48 h in all patients except those greater than 55 with a grade I injury and demonstrated a 97 % splenic salvage rate [47]. Leeper and colleagues developed a protocol of repeat CT imaging at 48 h after a sentinel event, which was associated with a decrease in the failure of NOM from 12 % to less than 1 % [48]. They recommend early repeat imaging to improve detection of SPA and AE, which can then be managed with SAE.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







