
Fig. 21.1
(a–c) Algorithm for management of suspicion of BDI
Epidemiology and Background
Definitions
BDI is a broad term used to describe a transection of, an excision of, a leak from, or a stricture of the biliary tree. Transection is the division of any duct in the extrahepatic or intrahepatic tree that results in loss of communication between segments of biliary branches. Excisions are identical to transections with the exception of a loss of an indeterminate amount of the bile duct. Both injuries could result, but not necessarily so, in drainage of bile into the peritoneal cavity, i.e., bile leak. Stricture is another problematic injury and does not include bile drainage into the peritoneal cavity; however, biliary communication with ducts downstream of the stricture is prevented or hindered by the narrowing of the afflicted ducts and consequent dilation of the upstream ducts.
The Strasberg Classification System
There are a number of systems that have been used to classify BDIs. Such systems are important for understanding how the injury occurred, for management decisions, and for long-term reporting of outcomes. Of the various methods used to classify BDI, we recommend the Strasberg classification because it provides a reasonable approach to treatment based upon the type of injury [10]. The Strasberg classification organizes BDIs using key features and is used in this chapter to help direct the physician in a predetermined care pathway.
The Strasberg classification categorizes BDI from A through E (see Fig. 21.2, Table 21.1). Type A through D injuries categorize leaks, lateral injuries, occlusions and transected minor ducts. The defining characteristic of these injuries is the intact communication between the intrahepatic ducts and the duodenum. However, this communication is lost in type E injuries, which are further subclassified into E1–E5. Each of these subcategories is indicative of a loss of direct communication between the intrahepatic ducts and the duodenum. This separation of the liver from the duodenum can be because of duct stenosis, complete occlusion of the duct or because of loss of ductal tissue as a consequence of duct resection. E2, E3, and E4 injuries are the most common type of BDIs that occur during laparoscopic cholecystectomy [10, 11].


Fig. 21.2
The Strasberg classification system for biliary injury
Table 21.1
Strasberg classification of bile duct injury
Type of injury | Description | Examples | Presentation | Treatment | |
|---|---|---|---|---|---|
A | Bile leak from a transected minor duct or cystic duct that does not disturb the continuity with the common bile duct | Transected small duct of Luschka from gallbladder fossa or cystic duct leak | 1.Pain 2.Fever 3.Sepsis 4.Mild hyperbilirubinemia 5.Biloma or biliary ascites 6.Possible peritonitis | Clip or ligate if operated upon or endoscopic stenting | Maintains continuity between the central biliary tree and duodenum |
B | Ligation of aberrant right posterior sector duct or aberrant segment duct VI or VII | Occlusion of aberrant right hepatic duct using a clip | 1.Asymptomatic elevation in AST, ALT, and alkaline phosphatase with normal bilirubin 2.Late presentation as pain or segmental cholangitis in obstructed liver segment 3.Liver atrophy of proximal liver segment or right posterior sector 4.Compensatory hypertrophy of left lobe or right anterior | Observation initially | |
C | Bile leak from a duct not in communication with the common bile duct | Transection of an aberrant right posterior sector or segment duct | Same as A injury | Ligation, drainage only, or RY hepaticojejunostomy | |
D | Lateral injury to major ducts of the extrahepatic biliary tree | Laceration or tear of the CHD, necrosis of lateral bile duct wall from cautery | Same as A injury | Primary repair, T-tube, or RY hepaticojejunostomy | |
E1 | CHD stricture or occlusion >2 cm below the biliary bifurcation | Resection/ablation with cautery; stenosis above the cystic duct junction with at least 2 cm of CHD before the bifurcation | Obstructive jaundice if total occlusion and no leak. Signs and symptoms similar to Classes A, C, D if leak present from proximal duct(s) | RY hepaticojejunostomy, bilateral hepaticojejunostomy, or right hemihepatectomy with left hepaticojejunostomy if serious vascular injury to R lobe | Disruption between major biliary tree and duodenum |
E2 | CHD stricture or occlusion within 2 cm of the biliary bifurcation | Resection/ablation with cautery; stenosis above the cystic duct junction with less than 2 cm of CHD before the bifurcation | |||
E3 | Injury to common hepatic duct and biliary confluence with preservation of the back wall | Resection/ablation with cautery; stenosis at the bifurcation of the right and left hepatic ducts resulting in no CHD | |||
E4 | Injury to the confluence including the back wall resulting in the loss of communication between right and left hepatic ducts | Resection/ablation with cautery of the CHD including the bifurcation | |||
E5 | Common hepatic duct and right duct injury | Resection/ablation with cautery of the hepatic duct along with injury to aberrant right duct |
Etiology, Incidence, and Pathogenesis
Iatrogenic injury, through operative trauma, is the main cause of BDI, accounting for 96% of all BDIs. Injuries arise through mechanical causes or anatomical misidentification. Mechanical causes of BDI vary from direct injury via inadvertent transection, excision, or clip application, to indirect injury caused by excessive traction or cautery. Anatomical misidentification is commonly associated with mistaking the common bile duct for the cystic duct or an aberrant duct, which has been termed the “classic injury” [12]. Any operation performed in the right upper quadrant of the abdomen poses a risk for BDI. Operative procedures such as gastrectomy, pancreatectomy, hepatic resection, or exploration of the common bile duct (be it via endoscopic, laparoscopic, interventional transhepatic, or open approach) have been implicated in BDIs. However, the highest risk procedure for operative biliary injury is cholecystectomy, with the laparoscopic approach being the leading cause [13].
Laparoscopic cholecystectomy is known to provide many benefits, namely, less pain postoperatively, earlier return of bowel function, fewer cosmetic defects, shorter length of hospital stay, earlier return to full activity, and decreased overall cost in comparison to open cholecystectomy [14–16]. However, the incidence of BDI has dramatically increased with the adoption of laparoscopy as the method of choice for gallbladder removal. The traditional open approach to cholecystectomy demonstrated a stable 0.16–0.2% rate of BDI [10, 17]. This is in stark contrast to more recent data suggesting the incidence of BDIs resulting from laparoscopic cholecystectomy to be between 0.3 and 0.7% [18]. These injuries, according to two recent studies, are more often complex, Strasberg class E (Table 21.1) [11, 19].
The relative low incidence of BDI during cholecystectomy is magnified by the volume of cholecystectomies performed nationally. In the United States, there are approximately 750,000 cholecystectomies performed every year, making it the most common abdominal operation [18]. This number reflects not only the burden of gallstone disease on the US population, but also the onus on surgeons to recreate a procedure with exceptional high standards and to practice preventive methods to avoid BDIs. Given the high volume of cholecystectomy and incidence of biliary injury, it is estimated that between 2,250 and 5,250 BDIs occur each year.
Prevention and Avoidance
Avoidance of BDIs requires a change in the way a surgeon manages cholecystitis, prior to operating, and how he/she manages a suspicion of BDI, once in the operating room. The latter is discussed later in this chapter. Dr. Steven Strasberg has stressed the importance of “changing the culture of cholecystectomy” as a means to teach surgeons how to reduce BDIs during cholecystectomy [20, 21]. In his article, he encourages surgeons to constantly assess ways to prevent entering into situations that increase the chance of causing a BDI. An example of such a circumstance is when operating on a severely inflamed gall bladder. In such settings, cholecystostomy tube and antibiotics may be a safer option [22–24]. While retrospective studies have concluded that operating on a severely inflamed gallbladder may be safe, these were uniformly underpowered to assess BDIs and it is the experts’ opinion that in such setting BDI is more likely and a surgeon should consider drainage procedures as part of the treatment pathway [20, 21, 25].
Once in the operating room, achieving the critical view of safety, prior to duct or artery transaction, is universally recognized as the safest and most appropriate manner of avoiding BDI during cholecystectomy [10, 26–28]. Unfortunately, the critical view of safety cannot be safely obtained in a large percentage of patients because inflammation, bleeding, or anatomic variations preclude safe dissection at the neck of the gallbladder. When this situation occurs, the ACS should adopt alternative techniques for addressing the gallbladder disease. Such techniques include early cholangiography through the gallbladder, cholecystostomy tube, conversion to open cholecystectomy, fundus first approach, partial cholecystectomy, and asking for assistance from another experienced surgeon [29]. Failure to employ these techniques, and proceed with dissection when there is unclear anatomy or inability to achieve a clear critical view of safety is ill advised and increases the risk of causing a BDI.
Clinical Presentation
Approximately 20–30% of BDIs are appreciated during the index operation [10, 30, 31]. The type of injury and the surgical approach used are factors in predicting early or late recognition. Major injuries to the main bile duct, those classified as types D and E, are identified intraoperatively one quarter to one half of the time [10, 32]. Type A and B injuries, however, being more subtle in nature, are rarely identified during the index operation [10]. The timing of recognition also differs in open versus laparoscopic cases. As may be expected, open cases that result in biliary injury are more likely to be appreciated during the index operation that those than occur during laparoscopic procedures [32].
A BDI should be suspected or placed high on the differential diagnosis of any patient following a recent operation in the right upper quadrant who presents in one of two ways. The first is with elevation of liver function tests. More specifically, total bilirubin and alkaline phosphatase levels will incrementally elevate beyond the patient’s normal levels. The second presentation is due to symptoms caused by bilious drainage within the peritoneal cavity. This can result in a biloma or become diffuse in the peritoneal cavity resulting in biliary ascites or bile peritonitis.
Patient Characteristics
Patient demographics tend to mirror that of those that require procedures on or around the biliary tree. A 2005 report compiled patient, institutional, and outcome characteristics from 1991 to 2000 on patients who required reconstruction after sustaining a BDI resulting from laparoscopic cholecystectomy [3]. This series found that about 2/3 of injuries occur in female patients with an age range of 40–75 years old. A common misconception is that most patient with BDI present via the emergency department. The fact is that only about 35% of patients with BDI present through the ER while 61% present in other medical settings (clinics, offices) and a small proportion (2.5%) are transferred from other institutions [3]. Emergency cholecystectomy is associated with a higher rate of BDI (57%) compared to elective surgeries (31.8%). Upon discharge, the majority of patients with BDI (74.9%) are routed home, while 19.6% are transferred to short-term hospitals or skilled nursing facilities [3].
Bile Duct Injury Diagnosis and Patient Management
It is important to note that proper management of BDI starts prior to definitive diagnosis of an injury. Ignoring the suspicion of BDI is in fact often the main cause in patient deterioration, and often, cause for medicolegal complications (see section on “Litigation”). The ACS is advised to treat any deviation in a patient’s expected postoperative recovery as a potential BDI until proven otherwise.
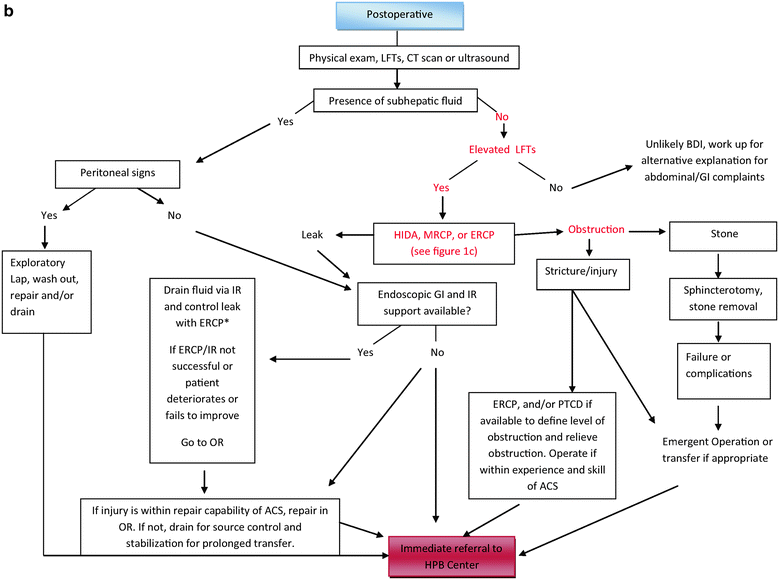
Suspicion of BDI can occur intraoperatively (surgeon’s intuition, presence of bile, unexpected anatomy of structures) or postoperatively (deviation from normal postoperative recovery pathways). The exact management pathway an ACS follows when such suspicion arises is influenced heavily by several factors: surgeon factors (his/her experience level), setting factors (resources at hand included IR and GI support), and patient factors (patient stability and comorbidity and patient and family wishes). This section and the algorithm presented in Fig. 21.1a–c should help the ACS navigate these variables to achieve a favorable outcome. Any surgeon that is in the position of having to manage BDIs is encouraged to understand these factors and assess their personal ability, their operating room’s equipment, their local and regional resources, and their comfort level with managing BDI repair. The surgeon should develop a management plan for addressing BDI that is specific to their situation and ensures the best possible recovery of the patient.
Intraoperative BDI Suspicion
BDI avoidance extends into the operating room before and after suspicion that such an injury had occurred (Fig. 21.1a). Intraoperatively, the avoidance of BDI starts by halting further progression in the operation once a suspicion of BDI exists. Often progression would result in a worst BDI due to the surgeon being in the wrong plane and, in the attempt to reenter into the correct plan, cause biliary or vascular structural injuries. Thus, as soon as a BDI is suspected intraoperatively, the procedure stops and the management of the possible injury begins.
In such a setting, the surgeon is advised to employ methods to better define the anatomy, such as intraoperative cholangiography (IOC) or asking for a second surgeon to scrub in and assist in the assessment and management of the injury. A properly performed IOC that clearly shows all parts of the biliary tree (duodenum, CBD, CHD, CD, right anterior section, right posterior section, and left liver) should reassure the surgeon to proceed with the procedure. An IOC that is not complete, i.e., does not show all parts listed, can easily be misinterpreted and the ACS is advised not to ignore his/her suspicion and utilize techniques to complete the IOC (see section on “Cholangiography”) or ask for a second opinion. The ACS may also request radiology to formally read an IOC if there is any doubt in biliary or hepatic anatomy.
If a BDI is demonstrated, then the surgical expertise of the operating surgeon for managing BDI or that of an in house surgeon, dictates the next step in management. It is important to note that BDIs occur across a spectrum and while expertise and skill may exist to manage low BDIs (Strasberg A, D, E1), high injuries (Strasberg E2–E4), posterior sector duct injuries (Strasberg B, C, E5) [33–35] and/or vasculobiliary injuries [36, 37] require a different set of expertise and hepatobiliary capability that typical does not exist except in specialized hepatobiliary units.
In the event that repair of a particular BDI is beyond the surgeons’ or hospital’s ability, immediate phone contact with a HPB referral surgeon or center is advised to ensure the best patient outcome. This is best accomplished immediately from the operating room. Again, these referral patterns are best established in advance. If immediate phone conversation is not possible, the surgeon is advised not to proceed and to establish good drainage of the injury, talk to the family and patient, and refer the patient with all documentations, images, and ensure surgeon–surgeon phone conversation in a timely manner. The use of a referral checklist (Table 21.2) is recommended to avoid errors of omission and affect a safe transfer to a higher level of care hospital and HPB surgeon.
Table 21.2
Transfer checklist
□Communicate with patient and key family members rational and need for transfer | |
□Name of receiving physicians and hospital | |
□Medical records/chart | |
□Key laboratory tests | |
□Current medications | |
□Brief note on physical/physiologic status | |
□Patient cognition, awareness of situation □Urine output □IV access □Drains—clearly label, secure □Pain control plan | |
□All pertinent imaging studies on a disk | |
□Operative note | |
□Define the biliary injury as understood | |
□Hand drawn figure portraying injury/anatomy and position of drains □Cholangiograms □Send intraoperative digital picture if possible | |
□Surgeon to surgeon communication | |
□Before transfer □Follow-up communication plan |
If the type of BDI lends itself to immediate intraoperative repair, i.e., absence of a vascular injury and the presence of sufficient surgical expertise, such repair should proceed based on best standards for such injury. In this situation, the surgeon is advised to leave the operating room, if safe, in order to inform the patient’s family or whomever has accompanied them to the hospital that an injury has occurred and additional surgery is going to occur in an effort to repair the injury. Immediate disclosure to the patient about the nature of the injury and repair should occur as soon as the patient is awake and alert. Caution is advised in ignoring an injury to the right hepatic artery or an accessory right hepatic artery or in underestimating the level of injury because immediate repair in such setting may result in poor long-term outcomes [5, 38, 39].
Postoperative Suspicion of BDI
The ACS may be confronted with a patient with complications of BDI in the immediate postoperative period or months to years after a previous right upper quadrant operation (Fig. 21.1b). Patients presenting in the immediate postoperative period (e.g., less than 30 days) will usually give a history of never having felt well in the postoperative period and usually show signs of acute illness. The evaluation and management of these patients is discussed in detail below. In contrast, patients who present with a bile duct problem months to years later usually have an insidious onset of symptoms that is related to an underlying bile duct stricture, which manifests itself in the form of abdominal pain, jaundice, and/or cholangitis. These strictures may be secondary to an unrecognized injury made at the time of operation related to a clip or cautery damage to the bile duct. Alternatively, a bile duct stricture may have evolved following BDI that was addressed in the immediate postoperative period from reoperation, endoscopic or percutaneous management. These patients should be referred to a surgeon and/or endoscopist experienced in the management of biliary strictures. Discussion of these patients is beyond the scope of this chapter and the interested reader can read about the approach [40–42].
As stated previously, suspicion of BDI should occur when any patient undergoing RUQ surgery complains of abdominal problems in the early postoperative period or has an unexpected postoperative course. A prompt and thorough evaluation of these patients to exclude BDI is indicated. This evaluation should include physical examination, laboratory studies (to include liver function tests), and abdominal imaging. While this evaluation is being initiated, the patient should be resuscitated and broad-spectrum antibiotics initiated. An important early step in the evaluation process of a presumed BDI is for the surgeon to determine if the patient has an active bile leak as part of their biliary injury or if they have a ligated or clipped duct without a leak. This is important because if a bile leak exists, surgical site control of the leak becomes the next priority (see later section on “Source control of bile leak”). If a leak does not exist, the management follows a different pathway (Fig. 21.1b).
Abdominal imaging is best achieved by either ultrasound or CT scan. If ultrasound is performed, the sonographer should be asked to interrogate the arterial and portal flow to the right lobe of the liver as well. In many patients ileus, obesity or intestinal gas may prevent clear views of the subhepatic or subdiaphragmatic spaces by ultrasound. For this reason, an abdominal/pelvic CT scan with iv and oral contrast provides the most information because it can identify fluid collections anywhere in the abdomen as well as provide anatomical detailed information about the critical structures in the porta hepatis as well as integrity of arterial and portal blood supply to the liver. However, I.V. contrast should not be given to a patient if they are dehydrated or have an elevated creatinine.
A bile leak will usually lead to diffuse ascites or loculated fluid collections. It is important to note that patients with an active leak with large amounts of bile in their peritoneal cavity may have little to no tenderness while others with a small amount of bile in the peritoneal cavity may have peritoneal irritation. So the absence of peritoneal signs on examination does not exclude a major bile leak and the acute care surgeon should not be lulled into complacency regarding the potential dire consequences of an active bile leak, when a patient does not yet have systemic illness or peritoneal irritation. An active bile leak that is walled off from the peritoneal cavity (usually in the subhepatic or subdiaphragmatic spaces (biloma)) often is not accompanied by abdominal tenderness. It is important to also understand that many patients with a biliary leak from BDI can also have partial or complete obstruction of other parts of their biliary tree.
If no fluid collections or ascites is found on CT or ultrasound, and the patient has normal liver function tests, BDI is probably not the cause of the patient’s symptoms and physical findings. In such circumstances, alternative causes of the patient’s complaints need to be further investigated.
Alternatively, if no fluid collections or ascites are found on CT or ultrasound and the patient is jaundiced, or has elevated liver function tests, then it is presumed that the patient has biliary obstruction (follow red text in Fig. 21.1b). The most common cause of obstruction in the postoperative period would be a retained common bile duct stone, or an inadvertent clipped common or hepatic bile duct. A HIDA scan or ERCP can confirm the presence of obstruction and also exclude with confidence a bile leak. ERCP, if available, is the diagnostic test of choice as it cannot only identify the point of obstruction, but also because it can be therapeutic by relieving obstruction through removal of a stone or placement of a stent across a partially clipped but intact duct.
With an understanding of how BDIs occur, experienced HPB surgeons can usually predict the type of BDI a patient has after reviewing only the operative report, the CT or ultrasound and lab studies, and noting the presence, number and position of clips on abdominal radiographs or CT scan. This knowledge and understanding is also important for the ACS evaluating and managing patients with BDIs because it can alert them to specific types of BDI that they may elect not to explore in favor of early referral. A number of typical scenarios are provided to serve as examples of how various BDIs present in Table 21.3.
Table 21.3
Typical presentation scenarios of bile duct injury and the suggested management
Scenario 1 |
Marked elevation in bilirubin, alkaline phosphatase, AST, ALT No peritoneal fluid seen on imaging No peritoneal irritation or abdominal guarding Additional clips are seen in midline than cannot be explained if just the cystic duct and cystic artery were ligated. Clips positioned along the same vertical axis should be suspected to be on the common bile duct and hepatic ducts Possibilities: (1) obstruction from stone; (2) clip completely across bile duct (E1, E2) or right and left ducts (E4) with no bile duct transection; (3) complete transection of duct with complete occlusion of proximal biliary system (any E) |
Scenario 2 |
Mild elevation in liver function tests (bilirubin <3, and near normal alkaline phosphatase) with abdominal fluid present Abdominal tenderness may or may not be present Possibilities: Strasberg A, D, C or any E if one side of liver is not occluded |
Scenario 3 |
Mild elevation in AST, ALT, bili (<3). Moderate elevation in alkaline phosphatase. No fluid observed on CT. No leak demonstrated by HIDA with intact flow into small bowel. ERCP shows no obstruction or bile leak and is commonly interpreted as normal by endoscopist and radiologist. However, failure to fill a right posterior sector or segment VI or VII ducts is a partially clipped or unclipped transected right posterior sector duct (see Fig. 21.3) Probable: Strasberg B. Diagnosis confirmed by absence of right posterior sector or segmental ducts on ERCP, differential perfusion of right posterior segments VI or VII or right posterior section on a four phase CT scan, cholangiography filling right posterior sector ducts without filling of common bile duct |
Scenario 4 |
High bile duct injury suspected or observed Decreased perfusion to the right hemi-liver during the arterial phase of CT, or enhanced perfusion to the right hemi liver during the portal venous phase of CT scan is indirect evidence of right hepatic arterial ligation Marked elevation in AST, ALT without elevation in bilirubin shortly after operation Possibility: vasculobiliary injury |
Understanding some important physiologic facts can help the ACS properly interpret liver function tests in the setting of BDI. A mild elevation in bilirubin (<3 mg/dl) with otherwise normal LFTs or minimally elevated LFTs can result from bile in the peritoneal cavity. Marked elevation in serum bilirubin and clinical jaundice usually occurs only with complete bile duct obstruction. If one half of the liver is not obstructed, marked jaundice usually does not develop. However, serum alkaline phosphatase will become elevated with obstruction of just a single liver segment.
Once the presence or absence of a fluid collection and biliary obstruction is determined, the ACS needs to use his/her judgment as to the next best course of action depending upon a number of factors: his experience and comfort level of dealing with a BDI, the physiologic condition of the patient, availability of resources, and availability and time necessary for transfer to a tertiary center that can provide multidisciplinary HPB care.
We recommend that patients with diffuse intra-abdominal fluid collections accompanied with physical signs of peritonitis, or systemic toxicity be explored urgently once they are reasonably resuscitated. This is important, even if the ACS is not experienced in surgically correcting BDI for two reasons. First, the goal of exploration is drainage of bile collections, peritoneal toilet and surgical site control of the leaking bile duct(s). This is thoroughly and rapidly accomplished with exploration of the abdomen. Second, abdominal exploration is the best way to identify and treat a hollow viscous injury, which frequently accompanies biliary injury [43] and can be difficult to distinguish from BDI. In fact, a duodenal or small bowel perforation can be misinterpreted as a BDI and these injuries are not effectively managed with percutaneous drainage. In addition, failure to recognize, drain and control a hollow viscus injury in a timely manner (e.g., <24 h) is associated with substantially increased risk for death. Abdominal exploration can be initiated safely via a laparoscopic approach and in fact, is preferred in patients who were initially operated upon laparoscopically. The rational for starting abdominal exploration laparoscopically is in case the patient is found to have a simple cystic duct leak or type D injury that lend themselves to management without laparotomy.
An additional reason for exploring patients laparoscopically is when the ACS chooses to not repair the BDI and only drain it externally. Drainage is easily and rapidly accomplished and spares the patient the morbidity of a laparotomy incision just prior to hospital transfer. If for any reason, effective irrigation and drainage of the peritoneal cavity and a leaking bile duct cannot be accomplished laparoscopically, or if hollow viscus injury not confidently ruled out, the ACS should proceed to general laparotomy. By following this approach, the surgeon eliminates the devastating consequences of a missed bowel injury, rapidly stabilizes the patient by eliminating bile peritonitis and reduces the probability of the patient developing SIRS or organ failure as they are being prepared for transfer to a tertiary care center. If the hospital and acute care surgeon lack the ability to safely perform emergent laparoscopy or laparotomy, immediate contact and transfer to a higher level of care is advised.
While uncommon, a small bile leak can exist without a large fluid collection being detected by imaging. Therefore, if BDI is suspected for any reason, even in the absence of intra-abdominal fluid collections, a HIDA scan, MRCP, or ERCP can be obtained preferably in that order (from least invasive to most invasive) (see Fig. 21.1c). When the preliminary evaluation is normal, the patient should be resuscitated and monitored for at least a day with close follow-up once discharged from hospital.
In the setting of previous cholecystectomy, bile duct obstruction is presumed to be either a common bile duct stone or a ligated or clipped bile duct. If the patient had a non biliary right upper quadrant operation, and presents postoperatively with jaundice and/or subhepatic fluid collections, BDI should be considered as part of the differential diagnosis (Fig. 21.1c).
Source Control of Bile Leak
Once a bile leak is identified or heavily suspected, source control should be attempted emergently, even in stable patients in order to avoid the development of organ failure, SIRS and abdominal sepsis, through percutaneous, endoscopic or even operative drainage. If a patient is systemically ill, shows any signs of peritonitis, presents shortly after surgery (<48 h), or has diffuse ascites, we recommend emergent laparoscopy or laparotomy as opposed to percutaneous drainage. Alternatively, patients who present in a more delayed manner, are hemodynamically stable, have no peritoneal signs, and localized fluid collections, percutaneous drainage is appropriate and usually sufficient for source control of a bile leak.
The inexperienced surgeon should not endeavor to repair any BDI laparoscopically unless it is an easily identified cystic duct leak nor should they dissect in the porta hepatis in an effort to identify an injured bile duct for they could cause further damage and/or bleeding. The principle goal of the acute care surgeon at the time of laparoscopic exploration is to achieve source control of the leak as a means to stabilize the patient. Secondary goals of exploration are to identify the source and nature of the bile leak. If laparoscopy is not possible, or unsuccessful at evacuating blood clots and bile, then a small laparotomy through a right upper quadrant incision should be used to achieve source control and effective drainage.
Once a patient is resuscitated and stable, an effort to classify the type and level of BDI should be performed via ERCP, MRCP, or PTC. This is a critical step in the management of any BDI because bile leakage from a Strasberg class A, B, or C injury may be easily and appropriately managed by the acute care surgeon whereas a higher injury at or above the bifurcation may be beyond the capabilities of the surgeon and/or hospital. Even an experienced HPB surgeon must try to understand the level and complexity of a BDI prior to operation so that an appropriate and safe reconstructive operation can be planned and carried out. If ERCP, MRCP, and/or PTCD are not available and hence the nature and location of the injury cannot be determined preoperatively, the acute care surgeon must decide if the patient is stable for transport to a higher level of care or if emergent damage control laparotomy is warranted as a means of stabilization prior to transport. Source control can almost always be easily achieved no matter what type of injury via good external drainage. The type of BDI encountered coupled with the surgeon’s experience and judgment will dictate if the injury can be definitively managed at the time of exploration (e.g., simple cystic duct leak or small laceration of common bile duct) or if definitive repair should be deferred such as Strasberg class E or vasculobiliary injury. Based on the type of injury identified, the center specific pathways should define the best method of management. This may be endoscopic management (i.e., stent), percutaneous or surgical management if expertise and resources allow. Again, patients with a BDI at or above the biliary bifurcation, vasculobiliary injury, or who fail to respond to resuscitation should be immediately transferred to a center where multidisciplinary expertise exists in HPB disease.
Cholangiography
Intraoperative cholangiography is an essential tool in the ACS arsenal, and he/she are advised to know the exact ability of their operating room in advance [18, 44]. The ability to have live fluoroscopy is essential. Further, the ability to replay the fluoroscopy run is very helpful and essential for accurate determination of the presence BDI and level of injury [45]. The surgeon needs to also be comfortable in utilizing techniques that allow for better visualization of proximal ducts. These techniques are often necessary in patients with a history of sphincterotomy where obtaining enough intrabiliary contrast pressure to view intrahepatic ducts can be challenging.
The surgeon should not accept an incomplete cholangiogram as sufficient to alleviate suspicion of BDI. An improperly read cholangiogram, read as normal, in the presence of a BDI is a common phenomenon primarily because the cholangiogram is incomplete. A complete IOC must include visualization of the CBD, CHD, CD, left liver ducts, right anterior and posterior section ducts. It must also show passage of contrast into duodenum. Adjunct techniques to achieve a complete cholangiogram may be needed. These include placing the patient in Trendelenburg position, rotating the patient left or right, clamping the distal bile duct to facilitate proximal filling and using a smaller syringe to obtain higher injection pressure, using a balloon occlusion in the duct (with the ability to inject proximal and distal to the balloon), needle cholangiography via the common bile duct, duct irrigation with saline, and use of sphincter relaxants such as glucagon. Cholangiographic pictures and cine loops obtained intraoperatively are critically important information to transfer to the surgeon who ultimately manages the repair.
If immediate surgical repair is not performed at the time of the index operation, subsequent cholangiography can be performed via the subhepatic drains once they are well walled off from the abdominal cavity or via percutaneous transhepatic drainage catheters (see Fig. 21.3).


Fig. 21.3
Fluoroscopic image of right posterior section injury demonstrated via percutaneous drainage pigtail catheter (black arrow) cholangiogram. Note: straight endoprosthesis (white arrow) is in common bile duct with no communication with the right posterior sector duct injury
Repair Goals
The goals of biliary reconstruction are to provide definitive internal biliary drainage, to ensure long-term anastomotic patency with low chance of stricture and the avoidance of major postoperative complications such as bile leak, sepsis, organ failure and death. To achieve these goals, surgeons repairing BDIs should possess appropriate knowledge about biliary anatomy and injury, exercise solid judgment with respect to the timing of repair, the choice of repair and possess sufficient experience and technical skill in biliary surgery [9, 11, 19, 31, 46, 47]. An ACS who elects not to repair a BDI still plays a pivotal role in achieving the best possible outcome for the patient by timely diagnosis, stabilization through surgical site control, and early referral.
Basic Principles of Bile Duct Injury Repair
There are no set rules in BDI repair. However, there are general principles that a surgeon should follow as a means to achieve the best possible outcomes for the patient with BDI (see Tables 21.4 and 21.5). Optimal clinical outcomes of BDI repair begins with appropriate timing of the repair. The timing of surgical repair must always be individualized and will vary from patient to patient [9, 19, 46, 48]. As discussed earlier, the best possible outcome is achieved if a BDI is recognized at the time it occurs and repaired during the same operation [46, 49]. Unfortunately, for most patients, the BDI is not recognized at the time it occurs and the patient subsequently presents in the postoperative period. When an experienced HPB surgeon is confronted with a patient several days after BDI has occurred, he/she has to evaluate and use their judgment if an early versus a late repair should be performed. There is no high level medical evidence to support one approach over the other though many reports have shown that delayed repair results in excellent outcomes [48]. In fact, it is not realistic to perform a prospective, controlled, randomized trial to solve this question because of the variations in patients’ anatomy, inflammatory response, and type of injury. However, some basic criteria for proceeding with immediate repair are offered in Table 21.4 while other criteria for delaying repair are listed in Table 21.5.
Table 21.4
Conditions for going ahead with bile duct repair
•Physiologically stable |
•No systemic or localized infection |
•No significant edema of the bile duct or intestine |
•Absence of concomitant major vascular injury |
•Minimal or well controlled medical comorbidities |
•Complete cholangiography of all liver segments obtained |
Table 21.5
Surgical checklist for considering emergent repair
The following guidelines can serve as a surgical checklist for the surgeon who is faced with the challenge of repairing a bile duct. A yes answer to any of these questions is grounds for not performing repair, delaying repair, or transferring the patient to an experienced surgeon in biliary repair |
•Is there systemic or intra-abdominal sepsis? |
•Is the patient hemodynamically unstable? |
•Is there significant inflammation in the porta hepatis or bowel wall edema? |
•Is the level of injury at or above the bifurcation (Strasberg E2 or higher)? |
•Is there a right posterior sector duct injury? |
•Is there significant thermal injury to the bile duct close to the bifurcation or to any of the sector ducts? |
•Are the orifices of the hepatic duct or sector ducts <3 mm? |
•Is there evidence of concomitant vascular injury, such as ligation of the right or an accessory right hepatic artery? |
•Is the surgeon unable to delineate all liver segments with cholangiography? |
•Is the surgeon not able to identify healthy proximal hepatic duct below the bifurcation with good blood supply that is at least 5 mm in diameter? |
Patients satisfying the criteria for early repair (e.g., within 2–4 days of injury) can have excellent long-term outcomes when repaired by experienced HPB surgeons [50, 51]. However, when a patient satisfying these criteria is explored early and found to have significant inflammation of the porta hepatis and/or of their intestines or an unexpected vasculobiliary injury, long-term results of repair are less satisfactory and postoperative complications are high [36, 39, 52]. Under such circumstances the surgeon must decide to risk repair and accept a high rate of early and late failure or to delay repair, typically for 6 weeks to 3 months. This is a critically important decision that requires keen judgment and experience [2, 8, 31, 41, 47, 48, 51].
If the surgeon decides not to repair a BDI at initial laparotomy, then the right upper quadrant should be adequately drained via transabdominal drains placed in the subhepatic space. Also, when a patient has high biliary transection (e.g., Strasberg E2–E4), or very small ducts in whom percutaneous transhepatic catheters have not yet been placed, the orifices of the transected ducts can be cannulated with small pediatric feeding catheters (usually 5 French) which are secured to the edge of the transected duct and then exteriorized. These catheters can subsequently be used to obtain cholangiograms to help delineate the patient’s biliary anatomy and to serve as guides for future biliary reconstruction. Additional subhepatic drainage is always indicated in these patients because even with ductal cannulation and drainage, bile will leak around these catheters into the subhepatic space.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







