Chapter 94 Bacterial Infection, Antimicrobial Use, and Antibiotic-Resistant Organisms in the Pediatric Intensive Care Unit
It is important to treat infections aggressively to obtain the best clinical and microbiologic outcomes. Judicious use of antibiotics is also important to reduce antibiotic pressure on pathogens and reduce the creation of antibiotic resistance. This chapter reviews the most clinically important classes of antibiotics, including those currently under investigation and not approved by the U.S. Food and Drug Administration (FDA) for use in children. Many textbooks about infectious diseases have excellent in-depth reviews of antibiotic characteristics,1–3 and an annually updated review of all available, the FDA-approved document published by the American Society of Health-System Pharmacists.4 Mechanisms of antibiotic resistance are reviewed, as is antibiotic therapy designed to meet the challenge of currently isolated antibiotic-resistant organisms. Tissue penetration characteristics and dosing of the antibiotic are critical; pharmacodynamic characteristics of different classes of antibiotics against different types of pathogens often help determine the dosing regimen required for microbiologic and clinical cure.5,6 Inadequate dosing of antibiotics may actually facilitate the development of antibiotic resistance.7 Timing of the first dose of antibiotics is also critical, and delay may increase morbidity and mortality.8–10
Antibiotic Classes
β-Lactam Antibiotics
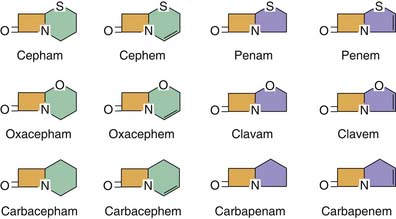
β-Lactam antibiotics are a diverse group of antibiotics. The β-lactam ring that characterizes these compounds is usually attached to a ring structure that defines the class of antibiotic agents as penicillins, cephalosporins, carbapenems, or monobactams (Figure 94-1). The β-lactam structure is thought to interfere with bacterial cell wall synthesis and repair by preventing transpeptidation and transglycosylation of the pentapeptide precursors in the formation of peptidoglycan in the creation of the cell wall.11 The target transpeptidase enzymes, also known as penicillin-binding proteins (PBPs), are vital for creating cell wall integrity to maintain the osmotic gradient between the organism and the external environment. The PBPs carried by different bacterial species have different structures, leading to differences in the binding affinity for various β-lactam agents. Each organism may actually produce several PBPs. As a class of antibiotics, β-lactam are bactericidal at concentrations up to 2 to 4 times the minimum inhibitory concentration (MIC) of the agent.
Cephalosporins
Cephalosporins can be distinguished on the basis of activity against gram-negative pathogens and stability of the antibiotic to a number of the gram-negative β-lactamases. The cephalosporins fall roughly into five categories (“generations”) on the basis of these characteristics. First-generation cephalosporins (cephalothin, cefazolin) are active against most strains of E. coli but are not entirely stable to the β-lactamases produced by some strains of E. coli and H. influenzae. The activity of these antibiotics against gram-positive pathogens such as S. aureus is close to that of oxacillin and nafcillin; however, none of the current cephalosporin antibiotics of any generation displays reasonable activity against the enterococci. Clinically relevant activity against B. fragilis does not exist for the first-generation cephalosporins. The second-generation cephalosporins (cefamandole, cefuroxime) have chemically increased intrinsic activity against gram-negative organisms, including E. coli and Klebsiella. They are also more stable against the principal β-lactamases of E. coli and H. influenzae. Activity against S. aureus is significantly decreased compared with the first-generation cephalosporins but is sufficient to achieve clinical success in many situations and to warrant FDA approval for treatment of these organisms. A slightly different group of antibiotics, the cephamycins (cefoxitin, cefotetan), have activity against the gram-negative enteric bacilli similar to the second-generation cephalosporins but display enhanced anaerobic activity against B. fragilis and may play a role in the treatment of intra-abdominal infections. Their activity against B. fragilis, however, is inferior to metronidazole, clindamycin, or the carbapenems.The third-generation cephalosporins, cefotaxime and ceftriaxone, have enhanced stability against the most prevalent β-lactamases of H. influenzae, E. coli, and Klebsiella and enhanced activity against many of the enterobacteriaceae but are, unfortunately, not stable to the inducible chromosomal β-lactamases (AmpC, type I) of Enterobacter, Serratia, or Citrobacter. Ceftazidime, another third-generation cephalosporin, has far greater intrinsic activity against Pseudomonas aeruginosa than previous cephalosporins, but it too is degraded by the inducible chromosomal β-lactamases of Enterobacter, Serratia, and Citrobacter, as well as the chromosomal β-lactamases of Pseudomonas. None of the third-generation cephalosporins are as active against S. aureus. Cefepime, a fourth-generation cephalosporin, has the best overall activity against both gram-negative and gram-positive pathogens, with activity against P. aeruginosa equivalent to ceftazidime and activity against S. aureus equivalent to second-generation cephalosporins. It is also the most stable to β-lactamase degradation by all but some of the ESBL class of β-lactamases. The novel fifth-generation cephalosporins, ceftobiprole and ceftaroline, are the first β-lactam antibiotics with activity against MRSA. Ceftobiprole was recently approved by the FDA for the treatment of complicated skin and skin structure infections in adults. Ceftaroline is currently in phase III clinical development. In addition to activity against MRSA, the fifth-generation cephalosporins have a spectrum of activity similar to that of third- and fourth-generation cephalosporins. Ceftobiprole also exhibits in vitro activity against enterococci and Pseudomonas. Ceftaroline is not reliably active against ESBLs, whereas ceftobiprole has activity against P. aeruginosa similar to that of cefipime. Although still in phase III trials, ceftobiprole has potential for use as empiric monotherapy for serious infections in the ICU because it has activity against both MRSA and P. aeurignosa.12
Carbapenems
Three carbapenems, imipenem (combined with cilastin, an inhibitor of a renal tubular dehydropeptidase enzyme, to avoid nephrotoxicity), meropenem, and ertapenem are currently FDA approved in pediatric patients older than 3 for treatment of complicated skin and skin structure infections (SSI), complicated intra-abdominal infections, and meningitis. Pediatric clinical investigation of doripenem, a fourth-generation carbapenem recently FDA approved for adults, is ongoing. The carbapenems have a β-lactam ring structure that differs slightly from the penicillins and cephalosporins (see Figure 94-1), with chemical modifications to enhance activity and stability similar to those of cephalosporins. The broad antimicrobial spectrum of activity of the carbapenems is similar and includes gram-negative, gram-positive, and anaerobic organisms. Relevant gram-negative pathogens include enteric bacilli such as E. coli, Klebsiella, Enterobacter, and Citrobacter in addition to P. aeruginosa. They are active against gram-positive organisms including S. aureus and streptococci, although activity against the enterococci is substantially less than penicillin G or ampicillin. Meropenem is slightly more active against gram-negative pathogens and imipenem is slightly more active against gram-positive pathogens, although there is probably no clinical significance in these differences for most infections being treated. Although not FDA approved for the treatment of nosocomial pneumonia, meropenem has been shown to be effective in numerous clinical trials. Both agents are active against anaerobes, including β-lactamase–positive strains of B. fragilis. Ertapenem has a similar spectrum of activity to the other carbapenems, although intrinsic activity against P. aeruginosa is less than that of the other carbapenems. Nevertheless, it has a prolonged serum elimination half-life compared with the other agents, allowing for once- or twice-daily therapy compared with three or four times daily. Doripenem was recently approved for the treatment of complicated intra-abdominal and complicated urinary tract infections in adults. Its spectrum of activity is similar to the other carbapenems; however, it appears to have more potent in vitro activity against P. aeruginosa.
With respect to toxicity, the carbapenems are well tolerated, although imipenem displays interference with central nervous system (CNS) γ-aminobutyric acid inhibition of neuron activity and was shown to be associated with an increase in seizure activity in children treated for bacterial meningitis compared with historic controls.14 On the basis of these observations, meropenem is the preferred carbapenem for children at risk for seizures or with CNS infections and inflammation. Meropenem is well tolerated in children. In pediatric trials, diarrhea and rash occur in less then 5%, and drug-related seizures were not observed in children with meningitis treated with meropenem.
Aminoglycosides
Aminoglycoside antibiotics are bactericidal in a concentration-dependent fashion against a wide range of aerobic pathogens. The first antibiotic in this class, streptomycin, was isolated from Streptomyces griseus and was first available in 1944. Subsequently, other aminoglycosides have been isolated from fungi, and chemical modifications to enhance activity and decrease toxicity have been made to older agents. These agents inhibit protein synthesis by irreversible binding to the 30S ribosomal subunit. The gram-negative spectrum of activity is extensive, including enteric bacilli (E. coli, Klebsiella, Enterobacter, Serratia), P. aeruginosa, and many free-living gram-negative bacilli that may only be pathogenic for immunocompromised children or those with trauma in which environmental contamination of deep tissues has occurred. These antibiotics have no clinically relevant anaerobic activity.
Although the first aminoglycosides exhibited substantial renal toxicity and ototoxicity, subsequent agents are significantly safer. With serum concentrations present within the therapeutic range, renal toxicity and ototoxicity are unusual. The most widely available parenteral agents are gentamicin, tobramycin, and amikacin. Because of the relatively low serum concentrations necessary to prevent toxicity and poor penetration into the spinal fluid, these agents are not used as primary therapy of CNS infections. Direct intrathecal instillation should also not be considered routinely for CNS infections because data collected during a prospective study of aminoglycosides as adjunctive therapy in neonatal gram-negative meningitis revealed higher rates of morbidity.15 Streptomycin, the most toxic of the aminoglycosides, continues to be used infrequently in the treatment of tuberculosis, plague, and tularemia in children.
Caution should be exercised in the use of these agents in undrained abscess infections, including intraabdominal infections. The acidic and anaerobic conditions present in abscesses produce MICs against aerobic gram-negative organisms that are 10 times higher than those documented under ideal laboratory conditions.16
Glycopeptides
Vancomycin is bactericidal against virtually all strains of staphylococci and against most strains of streptococci, although it is bacteriostatic against the enterococci. Resistance to vancomycin is noted to occur in strains of Enterococcus faecium (vancomycin-resistant enterococcus [VRE])17 and has now been described in S. aureus.18
Macrolides
Although erythromycin and related macrolides have been primarily used in the outpatient arena, they may be required in the PICU for children with severe pertussis or atypical pneumonia, or in children with extensive drug allergy precluding the use of standard anti-infective agents. The macrolides bind to the 50S ribosomal subunit of susceptible bacteria to prevent the formation of peptide chains, thereby inhibiting protein synthesis. Macrolides are primarily bacteriostatic at achievable tissue concentrations. Erythromycin is available for parenteral use as a lactobionate salt, whereas clarithromycin is only available as an oral agent. Azithromycin is composed of a 15-member ring structure and is considered an azalide. It is available in both oral and parenteral forms. In general, both clarithromycin and azithromycin are better tolerated than erythromycin because of the lack of degradation products seen with erythromycin that stimulate motilin receptors and lead to nausea, vomiting, and abdominal cramps. The macrolides have traditionally been used in the treatment of nonserious infections caused by group A streptococci, S. aureus, and Streptococcus pneumoniae. Both clarithromycin and azithromycin exhibit enhanced activity against respiratory gram-negative pathogens (e.g., H. influenzae). In addition, azithromycin has potential efficacy as a modulator of airway hyperresonsiveness,19 even in the absence of overt infection,20 that may have a role for children in the ICU with community-aquired pneumonia and exacerbation of underlying chronic lung disease or asthma.
Ketolides are a new class of antibiotics structurally similar to macrolides with enhanced bacterial ribosomal binding. Telithromycin is the first FDA-approved ketolide for treatment of community-acquired pneumonia in adults, including some multidrug-resistant strains of S. pneumoniae and group A streptococcus.21 Reports of significant hepatotoxicity with telithromycin22 use may limit further investigation in children. In addition, there is a “black box” warning for patients with myasthenia gravis.
All the macrolides demonstrate activity against Mycoplasma pneumoniae, Chlamydia, Legionella, and Bordetella pertussis. Macrolides are metabolized by cytochrome P450 enzymes, which cause potential drug‑drug interactions (see Chapter 118). These interactions have been well documented with erythromycin and clarithromycin but appear to be absent with azithromycin. Clarithromycin and azithromycin achieve high intracellular concentrations, with demonstrated efficacy against intracellular pathogens. Clarithromycin and azithromycin also demonstrate activity against a number of nontuberculous mycobacteria.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree