Fig. 3.1
Positionning of different automated weaning modes from intubation to extubation
3.1 ASV®
Adaptive support ventilation (ASV®) is the oldest automated MV technology and probably the most studied [17, 18]. It is fully automated and can adapt from intubation to the SBT. Its algorithm is based on the clinical information set by the user on the size and sex of the patient. The ventilator calculates the predicted body weight based on patient’s height (PBW) and then defines an ideal minute volume equal to 0.1 L/min/kg ideal body weight (e.g., 6 l/min for 60 kg of PBW). Then, the system initially uses the expiratory resistance and compliance to calculate the time constant. The ventilator uses the Otis and Mead equation [19] to provide specific minute ventilation (a combination of ideal tidal volume (Vt) and ventilatory rate (RR)) optimized for a minimal ventilatory work and the smallest energy expenditure. The clinician may then adjust three supplementary parameters:
percentage of the ideal expired minute volume, ranging from 50 to 250 % (resulting in a greater or lesser alveolar ventilation)
level of PEEP
FiO2 level
When the patient is passive (deeply sedated with no spontaneous breathing), the ventilator delivers pressure-controlled ventilation with Vt and RR previously calculated. Once the patient starts breathing and triggers the ventilator, the system tries to bring the patient to the ideal Vt/RR combination, if necessary by completing his or her ventilatory pattern with machine cycles. Spontaneous cycles triggered by the patient are delivered in pressure support mode (PSV). Finally, when the patient triggers spontaneously at a ventilatory rate greater than the targeted RR, the ventilator applies only pressure support. It gradually reduces the level of support it offers to shift the patient’s spontaneous Vt/RR combination toward the ideal curve, which represents all the possible ideal Vt/RR combinations. The principle is shown in Fig. 3.2.
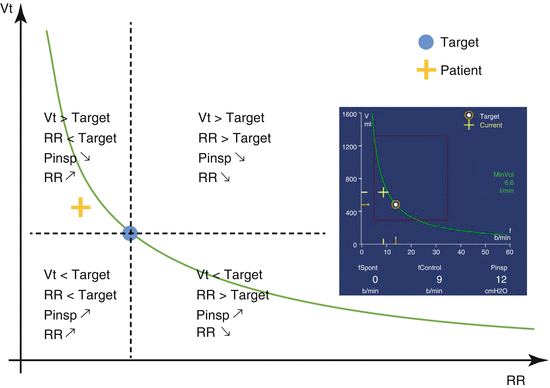

Fig. 3.2
ASV® simplified algorithm
3.2 SmartCare®/PS System
This system is a feedback loop centered on the weaning period. Its objective is to gradually reduce the level of pressure support while maintaining the patient in a “comfort zone.” It therefore requires the patient to be in PSV. It is based on the NeoGanesh expert system (from the Hindu god of wisdom and intelligence, Ganesh). The comfort zone is defined as
Vt > 300 mL
RR of 12–30/min
PETCO2 < 55 mmHg
SmartCare®/PS has certain requirements; it needs information about patient weight, type of airway humidification, type of tracheal prosthesis (intubation vs tracheotomy), existence of COPD or head trauma, and possible obstructive apnea syndrome. The ventilator must also be equipped with a capnograph. The ventilator evaluates the patient’s ventilatory status every 2–5 min (thus tolerating periods of transient worsening related to external stimuli). Patients are classified as shown in Fig. 3.3. The system modifies the level of pressure support and its alarms according to each diagnosed condition.
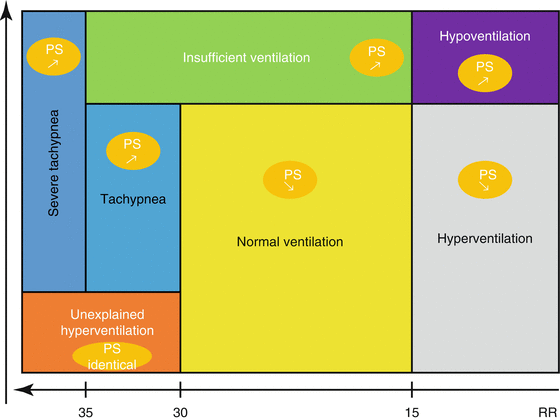

Fig. 3.3
Simplified algorithm of the SmartCare®/PS system
Once a period of stability is achieved with a level of pressure support that is low enough (depending on the threshold settings of the SmartCare®/PS) and if the patient has a PEEP less than 5 cmH20, an SB) is started. After 1–2 h of SBT, depending on the level of assistance set at the beginning of the SmartCare®/PS session, if the patient remains in stable condition, the system suggests the patient’s disconnection from the ventilator.
3.3 IntelliVent-ASV® System
The IntelliVent-ASV® system is the latest development of ASV®. It combines the latter mode with multiple feedback loops for setting PEEP and FiO2, with the same adjustment of a variable percentage of minute ventilation by collecting various biological signals from the patient (e.g., SpO2, EtCO2). With this system, the setting of PEEP and FiO2 is based on an algorithm defined by Hamilton. It is based on a combination of the lower PEEP table of the acute respiratory management (ARMA) of acute respiratory distress syndrome (ARDS) study for incremental PEEP and FiO2 situations, and the decremental scheme used in the Assessment of Low tidal Volume and elevated End-expiratory volume to Obviate Lung Injury (ALVEOLI) study (faster decrease of FiO2 than PEEP) when necessary [20, 21]. Furthermore, the continuous analysis of SpO2 changes induced by the MV provides another level of feedback. However, when preload dependency is suspected by the analysis of the SpO2 waveform, the optimization of the PEEP level can be limited to control hemodynamic effects of PEEP and increase safety [22]. Moreover, the measured level of EtCO2 has a negative feedback on the level of minute ventilation applied (i.e., the percentage of minute ventilation in the ASV setting). In addition, the latest version of the IntelliVent-ASV® has an automated SBT module (Quick Wean), which performs an SBT according to predefined criteria by the user (as with the SmartCare®/PS system) as soon as the level of assistance of the patient is low enough. This system thus offers a fully automated ventilatory strategy, from intubation to the SBT.
3.4 Review of the Literature
These automated systems are still poorly evaluated when compared with older conventional ventilatory modes. Regarding ASV®, a very modest benefit from a clinical point of view in postoperative cardiac surgery has been identified [23, 24]. Some studies show a reduction in the duration of ventilation ranging from 1 to 2 days in ICU patients [25, 26]. If there is a benefit in terms of duration of MV, it seems modest and of limited interest in the populations studied. The most important benefit would be to relieve medical and paramedical teams of the management of MV in the most “simple” patients. These ventilatory modes could also help enforcing the recommended guideline in the ICU by systematically applying them. However, more formal data are needed to confirm this.
Regarding SmartCare®/PS, data from the literature are conflicting. The results of two large studies by the team of Laurent Brochard [27, 28] found a 48-h reduction in the duration of ventilation and a 4-day reduction in ICU length of stay without deleterious effect in terms of reintubation. On the other hand, a large Australian study did not find any benefit of the SmartCare®/PS system when compared with a conventional weaning protocol [29]. However, the latter team had the benefit of a nurse-to-patient ratio of 1:1. To push the debate further, another study did not find any benefit over the use of a written weaning protocol in a population of surgical ICU patients [30], whereas a metanalysis by Friedrich et al. [31] found that weaning with SmartCare®/PS significantly decreased weaning time, time to successful extubation, ICU length of stay, and proportion of patients receiving ventilation for longer than 7 and 21 days.
Finally, concerning IntelliVent-ASV®, clinical assessment remains poor. Two recent studies have demonstrated the feasibility and safety of this ventilatory modality [31–33]. The authors found a much higher percentage of time spent in an optimal range (90 % vs 12 %) in the IntelliVent-ASV® group compared with management of the “usual” ventilation. These results were recently confirmed by Clavieras et al. [34] in an unselected ICU population. Moreover, an abstract was published in 2013 that included ARDS patients, thus confirming IntelliVent-ASV®’s safety in critically ill patients [35].
3.5 Conclusion
Novel automated ventilatory modes in the ICU look promising. Beyond their performance, their acceptance by health-care teams has yet to be evaluated [28]. Totally automated modes have initially focused on selected aspects of MV in ICU patients (initiation of the weaning process, or even its conclusion). They have shown a significant reduction in the length of the weaning process and have led to a novel, totally automated mode that needs further development and evaluation. The implementation of such modes in daily practice is a challenge for the future.
References
1.
Hess DR, MacIntyre NR. Ventilator discontinuation: why are we still weaning? Am J Respir Crit Care Med. 2011;184(4):392–4.PubMedCrossRef
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




