Fig. 16.1
12 Lead ECG with atrial fibrillation
Question
What is this rhythm?
Answer
Atrial fibrillation
This ECG demonstrates an irregularly irregular rhythm without discernable p-waves, consistent with atrial fibrillation (AF). The patient presented in cardiogenic shock. It was uncertain whether the AF was simply one manifestation of his decompensated heart failure or whether the onset of AF with a rapid ventricular rate was the primary reason for his decompensation, due to the rapid rate and loss of atrial contribution to ventricular filling. The duration of the arrhythmia was unknown, so the patient could not safely undergo elective cardioversion without anticoagulation and transesophageal echocardiography (TEE) to verify the absence of atrial thrombus. Because he was not hypotensive, immediate direct current cardioversion (DCCV) was not performed. Options for control of the ventricular rate were limited by his cardiogenic shock. He was admitted to the ICU where a heparin drip was initiated, and he was treated with intravenous furosemide. With diuresis alone, his shock state resolved, his creatinine, sodium, and lactate normalized, but his symptoms of dyspnea persisted. He underwent a TEE guided cardioversion, restoring sinus rhythm. He ultimately was discharged home with follow up with electrophysiology.
Principles of Management
Diagnosis
Conditions which increase the risk for new onset AF:
Triggers for Atrial Fibrillation
Acute/chronic pulmonary: pneumonia, pulmonary embolism, COPD exacerbation, sleep apnea
Heart failure, myocardial infarction, mitral valve disease
Cardiac or thoracic surgery
Acute or chronic hyperthyroidism, alcohol use
The incidence of AF increases steadily with advancing age. AF is commonly classified into three categories: paroxysmal, persistent (sustained longer than 7 days), or permanent [1]. Physical exam demonstrates an irregularly irregular heart rate on auscultation of the heart and palpation of the pulse. ECG findings include a variable R-R interval, with no discernable P-wave preceding each QRS complex. R-R variability may be less apparent at elevated heart rates (Fig. 16.2).


Fig. 16.2
R-R variability with very high ventricular rates
Echocardiograms demonstrate absence of A waves on pulse- and continuous-wave Doppler of the mitral valve in the apical views, along with a single E wave on M-Mode of the mitral valve in the parasternal long axis view (Fig. 16.3).
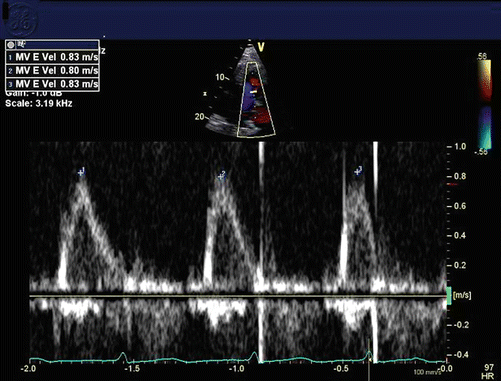

Fig. 16.3
Mitral inflow pattern in atrial fibrillation, with no atrial contraction, just passive filling (E wave only, no A wave)
AF may be asymptomatic or associated with a spectrum of symptoms ranging from palpitations to those of heart failure or cardiogenic shock, severe dyspnea, and lack of energy.
Physiologic Effects
The deleterious effects of AF come in two primary categories: hemodynamic embarrassment and cardioembolism. Hemodynamically, AF results in the lack of mechanical contraction of the left atrium, resulting in depressed preload of the left ventricle. In the setting of heart failure with reduced ejection fraction (HFrEF) or severe aortic stenosis, this acute loss of atrial “kick” can result in a meaningful decline in cardiac stroke volume and cardiac output [2]. Patients with heart failure with preserved ejection fraction (HFpEF) are exquisitely sensitive to preload so acutely lowering their preload conditions can have rapid deleterious effects on their cardiac function. The same principle applies to patients with hypertrophic obstructive cardiomyopathy (HOCM) and pulmonary hypertension [3]. In structurally normal hearts, some patients may be quite symptomatic from the loss of the atrial “kick”, and others may be asymptomatic. In many asymptomatic patients, their first sign of the arrhythmia is an embolic event such as a stroke [4].
A patient’s risk of embolic events such as cerebrovascular accidents (CVAs) or ischemic bowel can be calculated using a prognostic model such as the CHADS2-Vasc score that is available through a variety of online risk calculators (e.g. http://www.mdcalc.com/cha2ds2-vasc-score-for-atrial-fibrillation-stroke-risk/) (Table 16.1) [5]. This model has been validated and helps clinicians and patients understand the long term risk for embolic events [6].
Risk factor | Score |
|---|---|
Congestive heart failure/LV dysfunction | 1 |
Hypertension | 1 |
Age ≥ 75 y | 2 |
Diabetes mellitus | 1 |
Stroke/TIA/TE | 2 |
Vascular disease (prior myocardial infarction, peripheral artery disease, or aortic plaque) | 1 |
Age 65–74 y | 1 |
Sex category (i.e. female gender) | 1 |
Another model, the HAS-BLED score, uses similar inputs, but can help calculate the possibility of a severe bleeding event during anticoagulation (e.g. http://www.mdcalc.com/has-bled-score-for-major-bleeding-risk/) (Table 16.2) [7]. These models help predict risk of ischemic events over the course of years, so apply less to the acute inpatient management of patients with AF.
Letter | Clinical characteristic | Points awarded |
|---|---|---|
H | Hypertension | 1 |
A | Abnormal renal and liver function (1 point each) | 1 or 2 |
S | Stroke | 1 |
B | Bleeding | 1 |
L | Labile INRs | 1 |
E | Elderly | 1 |
D | Drugs or alcohol (1 point each) | 1 or 2 |
Treatment Strategies
Aligned with physiologic effects, treatment of AF has two main goals: hemodynamic and embolic risk mitigation. There are two potential strategies to mitigate the hemodynamic impact of AF rate control and rhythm control. Long-term outpatient management of AF was assessed in the AFFIRM trial and despite long-standing debate as to the applicability of the outcome, no significant mortality benefit to rhythm control was identified [8]. Inpatient management of AF is more guided by symptoms and clinical presentation.
Rate Control
Patients with AF frequently present with rapid ventricular rates which drive their symptoms. Heart rate control is achieved by two main classes of medications: beta-blockers and calcium channel blockers. Both classes of medications slow AV nodal conduction and exert negative inotropic effects. Care must be exercised with the use of these agents, particularly in patients with HFrEF, because of their negative inotropic effects. Diltiazem carries a greater risk of inducing cardiogenic shock and even death in patients with HFrEF, especially if they are in a decompensated state, versus metoprolol. Esmolol may be a reasonable option with very rapid offset that can be trialed in patients who may not tolerate rate controlling agents with negative inotropic effects. Digoxin has modest efficacy but is sometimes the best alternative when beta-blockers and calcium channel blockers are not tolerated. Extrapolation of data from the RACE 2 trial suggests that targeting a heart rate of less than 110 bpm is a safe management strategy, assuming hemodynamic stability (Table 16.3) [9].
Table 16.3




Common dosage of intravenous medications for rate control of AF
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







