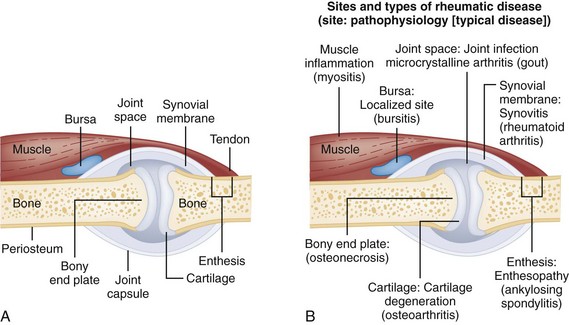
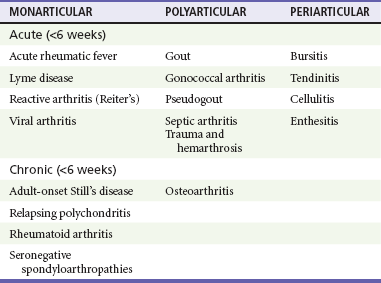
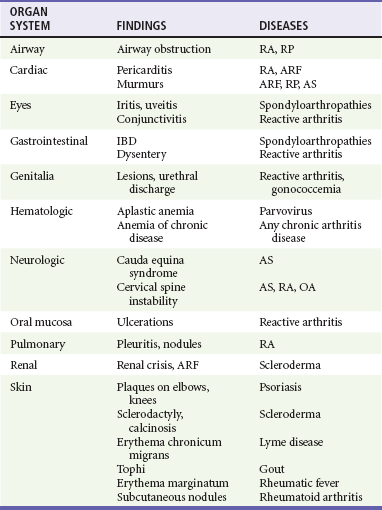
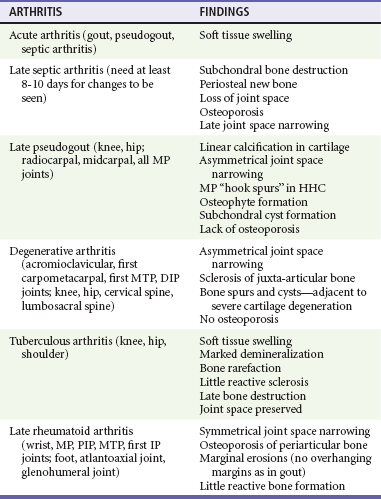
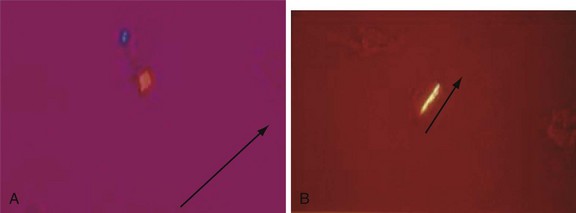
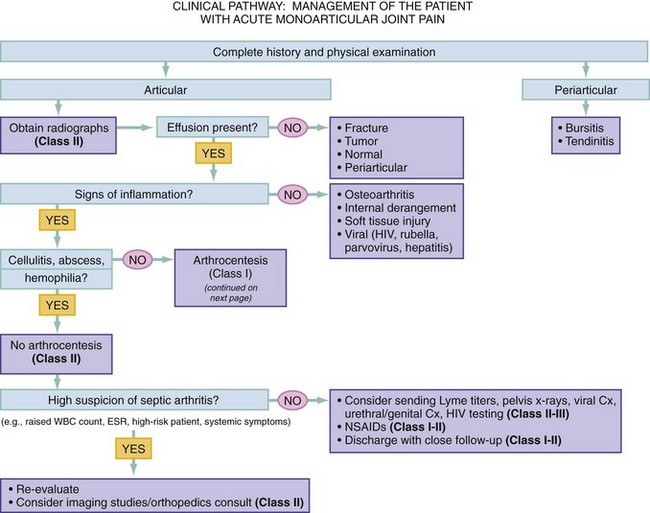
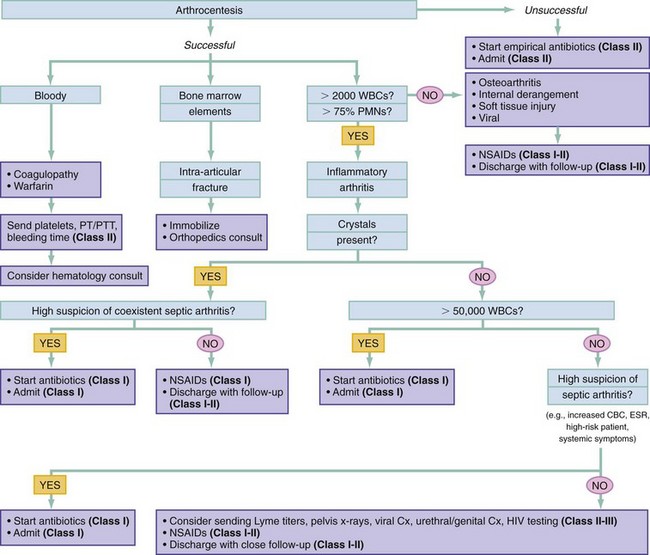
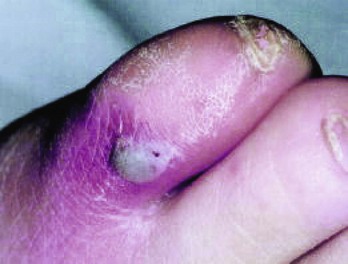
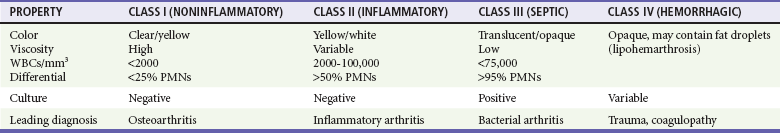
Chapter 116 Arthritis and its related disease states represent the most common cause of disability in the United States1; this is only expected to increase as the population ages and grows heavier. Because of the pain and limitations associated with joint inflammation, patients with complaints stemming from arthritis present frequently for emergent evaluation. Many of the arthritides are associated with premature mortality, and their treatments are associated with adverse effects. A systematic approach to evaluation of patients with arthritic complaints is important. The inflamed joint may be a diagnostic clue to a serious systemic illness (such as acute rheumatic fever or renal crisis), and unmasking of true arthritis emergencies can prevent or attenuate devastating disability. Arthritis and related conditions are among the oldest disease states described. Roman, Greek, and Egyptian cultures made reference to gout and rheumatoid arthritis and even associated these maladies with diet and socioeconomic status. Gout was called the disease of kings because of its relationship to alcohol and rich foods.2 Many celebrated figures in medicine, such as Hippocrates, Galen, and Sydenham, contributed to the description, classification, and treatment of rheumatic disorders.2,3 Reiter’s syndrome, however, takes its name from a discredited Nazi war criminal (many, including the physician who first bestowed the eponym in the English literature in1942, now advocate for the generic term reactive arthritis).4 Several world leaders through the ages suffered from arthritis, and some have argued that specific flare-ups have shaped history.2 Predisposing factors for gout, such as lead levels, may have influenced the rise and fall of civilizations.5,6 As opposed to synarthrotic suture joints of the skull and amphiarthrotic fibrocartilage unions like the pubic symphysis, the joints of concern in acute arthritis are the synovial or diarthrotic (moving) joints. These synovial joints are composed of two ends of subchondral bone covered by articular cartilage, surrounded by a capsule that is lined with a thin synovial membrane and supported by ligaments, tendons, and muscle (Fig. 116-1A). The pathologic process of arthritis can unfold during hours or years. Tissue destruction can be mediated by fast-acting catabolic pathways, or long-term changes to the composition of cartilage’s extracellular matrix may be brought on by abnormal loading patterns or trauma. The synovium plays a critical role in crystal deposition and inflammatory response (Fig. 116-1B). Pain is the hallmark complaint of patients with joint problems who come to the emergency department (ED) (Table 116-1). The pain may be acute or chronic (traditionally, more than 6 weeks) or an acute episode in a person with chronic disease. The patient may have had similar pain before, so it is important to know whether a diagnosis was previously made and what treatment, if any, was instituted. One must first determine whether the source of the inflammation or pain is articular or periarticular (outside the joint capsule). If the site of the patient’s pain is articular, the next step is to determine whether the arthritis is monarticular or polyarticular. The arthritis may be symmetrical (e.g., rheumatoid or drug induced) or asymmetrical (e.g., rubella, acute rheumatic fever, or gonococcal). In addition, it may also be migratory (e.g., gonococcal or rubella), subsiding in one area before presenting in another, or additive, remaining in the first joint and progressing to additional joints (Box 116-1). General Examination.: The physical examination searches for evidence of both local and systemic manifestations of rheumatic diseases (Table 116-2). Joints.: Joints are examined for warmth (the dorsum of the hand can detect a difference of 0.5° C), effusion, synovial thickening, deformity, range of motion, pain on actively loaded motion, and tenderness (generalized or localized, articular or periarticular). Spine.: The spine evaluation is best performed with the patient standing; the vertebral column is assessed for abnormal curvature or asymmetry. Although supporting evidence is scant, Schober’s maneuver is used to assess for the limitation of the lumbar spine motion that occurs in ankylosing spondylitis.7 Upper Extremities.: Localized tenderness and pain associated with active movement are more likely to be periarticular in origin. A shoulder affected by chronic arthritis or bursitis will have atrophy of the deltoid muscle. Generalized tenderness and pain, both at rest and with active and passive motion, suggest joint involvement. Lower Extremities.: Inflammation affecting the hip joint can be manifested as pain in the anterior thigh, knee, or groin. A hip joint effusion will cause the patient to hold the hip partially flexed. An externally rotated and abducted leg in a neonate strongly suggests infection.8 Range of motion of the hip is most easily tested by flexing the hip, bending the knee at a right angle, and rotating the heel medially and laterally to test for external and internal rotation, respectively. In the ED evaluation of arthritis, blood testing conveys only modest diagnostic value.9 The erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) serum level, white blood cell (WBC) count, and serum uric acid level are best used in the context of evaluating the likelihood of specific forms of arthritis; there are no guidelines or studies supporting the use of blood testing as a general screen of acute undifferentiated arthritis in the ED. Plain radiographs are more helpful in patients with chronic disease than in those with acute arthritis (Box 116-2). Common findings that help distinguish the different forms of arthritis are shown in Table 116-3. For lower extremities, weight-bearing radiographs can better evaluate joint space narrowing. Other radiologic modalities are occasionally performed as part of the workup of arthritis in an emergency setting. Ultrasonography compares favorably with plain radiography in the evaluation of joint effusions and synovitis associated with RA, and it is useful to evaluate pediatric hip effusion and to guide arthrocentesis.10 Magnetic resonance imaging (MRI) is well suited for imaging of cruciate ligaments of the knee, detection of early edema in periarticular structures and fluid collection in tendon sheaths, and determination of the extent of cartilage destruction.11 MRI is also the study of choice for diagnosis of osteonecrosis and is more sensitive than plain radiography for early osteomyelitis.12,13 The American College of Rheumatology guidelines recommend arthrocentesis to evaluate patients with an established history of arthritis who present with fever and new joint pain or effusion.14 Although scant literature on inoculated joint spaces exists, emergency arthrocentesis through overlying cellulitis is relatively contraindicated, and avoidance of the infected area during the puncture is recommended. Coagulopathy is the other relative contraindication, but arthrocentesis can be safely performed with a 20- to 22-gauge needle in the presence of international normalized ratios as high as 4.5.15 Arthrocentesis of prosthetic joints should be performed only to rule out infection and is best done in consultation with an orthopedic surgeon. Analysis of synovial fluid is essential for identification of crystalline and suppurative causes of acute arthritis (Table 116-4). Table 116-4 PMNs, polymorphonuclear cells; WBCs, white blood cells. From Fye KH, Morehead K: Joint aspiration and injection. In Imboden J, Hellman D, Stone J (eds): Current Rheumatology Diagnosis and Treatment. New York, McGraw-Hill, 2007:12. General Appearance.: Bedside inspection of synovial fluid for color, clarity, and viscosity can provide diagnostic clues. Normal synovial fluid is clear and colorless, with a viscosity that permits stretching of a “string” of fluid between the thumb and forefinger. Inflamed fluid is more opaque from an elevated WBC count, with a viscosity more like water because of enzymatic breakdown of glycosaminoglycans. Hemarthrosis is manifested after acute trauma or in the presence of coagulopathy, particularly with hemophilia. Lipohemarthrosis indicates ligamentous injury or an intra-articular fracture, and brownish synovial fluid may suggest the rare diagnosis of pigmented villonodular synovitis. Synovial Fluid Studies.: Routine laboratory analysis includes a cell count with differential, Gram’s stain, and crystal analysis; synovial glucose and protein concentrations are of little diagnostic assistance.16 A positive Gram’s stain is diagnostic, but a negative result for bacteria does not rule out septic arthritis; culture specimens should also be obtained. Blood culture bottles are more sensitive than other media at identification of pathogens.17,18 Synovial White Blood Cell Count.: Synovial WBC (sWBC) counts are often used to differentiate classes of synovial fluid; unfortunately, significant overlap exists between inflammatory and septic causes of acute arthritis.17 A very high fluid sWBC count or polymorphonuclear (PMN) cell pleocytosis indicates infection, but a more modest sWBC count or differential does not exclude it. The likelihood ratio (LR) for septic arthritis increases as the joint WBC count rises. According to one review, sWBC count of less than 25,000/mm3 = LR of 0.32; sWBC count of more than 25,000/mm3 = LR of 2.9; sWBC count of more than 50,000/mm3 = LR of 7.7; and sWBC count of more than 100,000/mm3 = LR of 28.0.9 However, low sWBC counts do occur early in infectious arthritis and in partially treated infections; high sWBC counts (>50,000/mm3) can occur in RA, gout, and pseudogout. Most of the cells in both septic and severe inflammatory arthritis are PMN cells. Crystal Analysis.: Although there is some variation in study findings, overall there is an acceptable inter-rater reliability in identifying monosodium urate and calcium pyrophosphate dihydrate crystals in acute arthritis.19 Crystal analysis is best performed by polarizing microscopy of a fresh drop of synovial fluid placed on a slide with cover slip (Fig. 116-2). Monosodium urate crystals are needle shaped and strongly negatively birefringent (yellow when parallel to the compensator and blue when perpendicular), ranging in size from 2 to 10 µm. Calcium pyrophosphate crystals, in contrast, are polymorphic, rhomboid, and positively (although weakly) birefringent. Lipid appears as a spherule with a Maltese cross, and hydroxyapatite is difficult to recognize. Inflammatory conditions of the periarticular soft tissues, such as olecranon bursitis, rotator cuff shoulder tendinitis, and prepatellar bursitis, can mimic monarticular arthritis, and classically polyarticular conditions, such as RA and the seronegative spondyloarthropathies, may present initially in only one joint. As a general rule, a patient presenting with monarticular arthritis has a septic arthritis until it is determined otherwise (Fig. 116-3).20 Epidemiology.: In two prospective series of patients presenting to the ED with monarticular arthritis prompting arthrocentesis, 8 to 27% of patients had bacterial joint infections.16,21 The incidence of septic arthritis in the general population is approximately 2 to 10 cases per 100,000 per year, with an age distribution curve for septic arthritis revealing bimodal peaks for young children and adults older than 55 years.22 Additional risk factors include low socioeconomic status, injection drug abuse (in which joint infections typically involve the axial skeleton but can involve extremities), alcoholism, diabetes, skin infections, advanced human immunodeficiency virus (HIV) infection or other immunocompromised states, chronic arthritis (particularly rheumatoid, crystalline, and degenerative OA), and following intra-articular corticosteroid injections or prosthetic implants. Pathophysiology.: Any diarthrotic joint, whether it is native, chronically diseased, or prosthetic, can develop septic arthritis. Bacterial pathogens infect the joint space most commonly by hematogenous spread, but direct inoculation and contiguous spread from bone or soft tissue infections also occur. Once in the joint, bacteria proliferate essentially unchecked in the highly vascular synovium, which has no limiting basement membrane. Bacterial components and toxins, as well as the inflammatory cascade they induce, trigger synovial proliferation with neovascularization and subsequent enzymatic, cellular, and cytokine degradation of articular cartilage. Septic arthritis, unless it is rapidly recognized and treated, results in serious disabling morbidity, with mortality ranging from 7 to 15% or higher.17 Septic arthritis can occur simultaneously with other forms of arthritis, especially RA and gout.20 The diagnosis of infectious arthritis in a patient with known crystal arthritis can be challenging because acute flare-ups of gout or pseudogout can cause fever, and crystals can emerge from an infected joint. For this reason, Gram’s stain and culture should be considered in select cases. Microbiology.: The microbiology of nongonococcal arthritis has remained fairly constant over time, except for the notable decline of Haemophilus and pneumococcal species in the postimmunization era.22 Acute nongonococcal septic arthritis in adults is caused most often by gram-positive organisms (75-90%), followed by gram-negative bacilli (10-20%) and then anaerobes, mycobacteria, and fungal and other unusual organisms.23 Overall, Staphylococcus aureus is still the most common cause of septic arthritis, with the rapid emergence of methicillin-resistant strains.24 Neisseria gonorrhoeae accounts for only 20% of cases of monarticular septic arthritis and more commonly is manifested with polyarthritis; it is discussed separately. Select populations have higher propensities for specific infecting organisms (Table 116-5). Table 116-5 Microbiology of Bacterial Septic Arthritis Related to Patient GNR, gram-negative rod bacteria. From Noble J, Greene HL: Textbook of Primary Care Medicine, 3rd ed. St Louis, Mosby, 2001. Prosthetic Joint Infection.: Prosthetic joint infections are classified as early (within a month of surgery) or late. Late infections can be caused by hematogenous spread from another infection or by indolent organisms introduced at surgery that may not surface for up to a year.25 Because the joint is literally replaced, antibiotic bioavailability and host immune response are both impaired in the prosthetic environment. A wide range of culprit organisms have been identified26; methicillin-resistant S. aureus (MRSA) incidence is rising.27 Arthrocentesis diagnosis of a suspected prosthetic joint infection is best done in consultation with the operating surgeon. A fluid WBC count of more than 1100/mm3 or a pleocytosis of greater than 64% PMN cells is sensitive and specific for infection in this setting.28 Clinical Features.: Patients with septic arthritis present with fever, joint pain, and effusion, typically in a single large joint (the knee is most common). Moderate fever occurs in 50% of cases17 but may be less common in the presence of advancing age or immunosuppressive states.17 Rigors and chills are reported in only 20% of patients. Polyarticular presentation occurs in 20% of cases, particularly in patients with RA or chronic joint disease, meningococcal infections, or overwhelming sepsis.17 Other physical signs, such as edema, tenderness, and decreased range of motion, have not been adequately evaluated for their prognostic value. Diagnostic Strategies.: Laboratory evaluation typically includes complete blood cell count, ESR, and CRP level, although in one study a WBC count above 10,000/µL and an ESR above 30 mm/hr only minimally increased the likelihood of septic arthritis.28 Furthermore, systematic reviews show insufficient evidence for a normal CRP level, ESR, WBC count, or procalcitonin level to rule out septic arthritis.29,30 Serum blood cultures reveal the causative organism approximately 25 to 50% of the time.31,32 The only definitive diagnostic test for septic arthritis is synovial fluid analysis. The fluid WBC count is directly proportional to the probability of a septic joint. Polymorphonucleocyte concentrations above 90% are also associated with an increased likelihood for septic arthritis. Synovial lactate levels greater than 5.6 mmol/L have an LR+ of 2.4 to infinity; smaller values make septic arthritis unlikely.33 Synovial lactate dehydrogenase (LDH) levels above 250 U/L are sensitive for septic arthritis, according to one study,16 whereas LDH levels below this seem to exclude septic arthritis. Low synovial glucose and high protein concentrations are neither sensitive nor specific for septic arthritis.32 Gram’s stain will show bacteria in 50 to 80% of infected joints.17 Synovial fluid cultures for both aerobic and anaerobic organisms should be performed. Management.: The key to successful treatment of nongonococcal septic arthritis is early diagnosis. Substantial delays in diagnosis directly worsen prognosis. Empirical antibiotic therapy is based on Gram’s stain or the presumptive consideration of likely organisms. Once the diagnosis is made, hospital admission is indicated for administration of intravenous antibiotics and needle, arthroscopic, or open drainage of the affected joint.34 Whereas the utility of performing or omitting joint drainage has not been prospectively studied, retrospective reviews suggest good cure rates with drainage, with outcomes dependent on initial severity.35 More severe cases require frequent, potentially daily joint aspirations. There are no randomized controlled trials of antibiotic regimens in septic arthritis. Antibiotic selection is initially based on Gram’s stain results and then adjusted on the basis of final culture results and sensitivities. For gram-positive organisms, the initial drug of choice is vancomycin 30 mg/kg daily in two divided doses as MRSA is frequently causative.24 For gram-negative bacilli, use a third-generation cephalosporin, such as ceftriaxone 2 g IV once daily, cefotaxime 2 g IV three times a day, or ceftazidime with gentamicin (especially if Pseudomonas infection is suspected). Although no trials of antibiotic duration have been reported, antibiotic therapy is generally continued parenterally for 2 to 4 weeks, depending on the response, and followed with 2 to 6 weeks of oral antibiotic therapy. Principles of Disease.: Gonococcal arthritis was considered the most common infective arthritis in the United States in the 1970s and 1980s and remains the most common form of joint infection in the sexually active population. In recent years, the incidence has decreased, most likely because of a general decline of N. gonorrhoeae prevalence, particularly of the more virulent strains.36 Host risk factors for dissemination of gonococcal infection to joints include pregnancy, menstruation, and complement deficiency. There is a 4 : 1 female predominance, possibly explained by mucosal infections more often being asymptomatic in women. Gonococcal arthritis represents a clinical and pathologic course distinctly different from that of other bacterial infections and is less likely to create long-term joint disease.25 Clinical Features.: Systemic gonococcal infection complicates 0.5 to 3% of mucosal infections and is manifested with two somewhat overlapping musculoskeletal syndromes. The first is a localized septic arthritis, more commonly oligoarthritis than monarthritis, predominantly in the wrist, knee, or ankle. The effusions may be modest. True disseminated gonococcal infection (sometimes termed arthritis-dermatitis syndrome) is manifested with bacteremia, diffuse migratory arthralgias, characteristic skin lesions, and tenosynovitis (Fig. 116-4). A similar syndrome has recently been recognized as a result of Neisseria meningitidis infection.37 Management.: Microbiologic diagnosis is a problem because both synovial and blood cultures are positive for gonococcus in no more than 10 to 50% of cases.25 The diagnostic yield is higher when specimens are plated on Thayer-Martin medium and even greater with the use of polymerase chain reaction. Synovial fluid often yields a positive Gram’s stain result, and the synovial WBC count tends to be lower than in nongonococcal arthritis (40,000-60,000 cells/mm3). Cervical, urethral, rectal, and pharyngeal cultures are positive in up to 75% of cases, so all mucosal orifices of the patient (and partner, if possible) should be cultured appropriately.36 In disseminated gonococcal infection, the skin lesions often contain the gram-negative diplococcus. The current Centers for Disease Control and Prevention (CDC) guidelines recommend hospitalization, especially if etiology or compliance is uncertain, and treatment with intramuscular or intravenous ceftriaxone 1 g every 24 hours or intravenous ceftizoxime or cefotaxime 1 g three times daily, with transition to oral cefixime 400 mg twice daily for at least a week.38 Partners should be evaluated. Presumptive treatment of Chlamydia is also advised.
Arthritis
Perspective
Principles of Disease
Clinical Findings
Patterns
Physical Examination
Diagnostic Strategies
Radiologic Tests
Computed Tomography, Magnetic Resonance Imaging, and Sonography
Arthrocentesis
Indications and Contraindications
Synovial Fluid Examination
Differential Considerations and Management
Septic Arthritis: Nongonococcal Bacterial
PATIENTS
ORGANISMS
Neonates and infants
Staphylococcus aureus, group B streptococcus, GNR
Children
Haemophilus influenzae, S. aureus
Adolescents and young adults
Neisseria gonorrhoeae, Chlamydia trachomatis
Older adults
S. aureus, Streptococcus, GNR
Sickle cell anemia
Salmonella
Injection drug abusers
Pseudomonas, S. aureus, GNR
Gonococcal Arthritis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Arthritis
Only gold members can continue reading. Log In or Register to continue