EPIDEMIOLOGY
The exact number of cases of shock that present to the ED in the United States is difficult to ascertain due to the insensitivity of clinical parameters, current definitions, and lack of a central database repository. Previous estimates propose that more than 1 million cases of shock are seen in the ED each year in the United States.1 These estimates are largely based on the assumption that hypotension, defined as a systolic blood pressure <90 mm Hg, is consistent with shock in adults. Using this definition, the incidence of patients with hypotension that present to American EDs is approximately 5.6 million cases per year.2
Mortality depends on the inciting event. Septic shock has an estimated mortality of 40% to 60%.3 Cardiogenic shock has an estimated mortality of 36% to 56%.4 Approximately 30% to 45% of patients with septic shock and 60% to 90% of patients with cardiogenic shock die within 1 month of presentation.3,4 With a greater recognition and improved treatment, mortality from neurogenic shock has been reduced significantly. The definition of and treatment approach to shock continue to evolve, but the initial approach to a patient in shock follows similar principles, regardless of the inciting factors or cause.
Patients present to the ED in varying stages of critical illness and shock. These stages are confounded by age, comorbidities, and delays in presentation. A focus on early recognition, rapid diagnosis, and empiric resuscitation is essential. Therapy and patient stabilization may need to occur simultaneously with evaluation.
PATHOPHYSIOLOGY
Shock is a state of circulatory insufficiency that creates an imbalance between tissue oxygen supply (delivery) and oxygen demand (consumption) resulting in end-organ dysfunction. Reduction in effective perfusion may be due to a local or global delivery deficiency or utilization deficiency with suboptimal substrate at the cellular or subcellular level.5 The mechanisms that can result in shock are frequently divided into four categories: (1) hypovolemic, (2) cardiogenic, (3) distributive, and (4) obstructive.
An understanding of the mechanisms of oxygen delivery and consumption is foundational to the treatment of shock. While the physiology is complex, familiarity with the basic principles, equations, and their interactions is essential (Tables 12-1 and 12-2). As noted in the cardiac output (CO) equation, CO is determined by heart rate and stroke volume. Stroke volume is dependent on preload, afterload, and contractility. The mean arterial pressure (MAP) demonstrates the impact that CO has on MAP (which can also be estimated with the formula: 2 × diastolic blood pressure + systolic blood pressure/3). This is important because there is a MAP threshold below which oxygen delivery is decreased. Systemic vascular resistance (SVR) directly impacts MAP, but also impacts afterload and thus CO. The physiologic mechanism of oxygen delivery to peripheral tissues (Do2) is described in the oxygen delivery equation. Recognize that blood pressure is not represented in this equation. Patients in shock may initially have normal blood pressures (cryptic shock), yet have other objective signs of shock (see “Clinical Features” below). It is from these basic equations that the concept of preload influencing stroke volume, which itself influences CO and Do2, has become fundamental in shock management.
| Parameter | Equation Specifics |
|---|---|
| Cardiac output | Cardiac Output = Heart Rate × Stroke Volume CO = HR × SV |
| Mean arterial pressure | Mean Arterial Pressure = Cardiac Output × Systemic Vascular Resistance MAP = CO × SVR |
| Oxygen delivery | Oxygen Delivery = Cardiac Output × Arterial Oxygen Content Do2 = CO × [(1.39 × Hb × Sao2) + (Pao2 × 0.0031)] Do2 is the amount of O2 delivered to the tissues per minute. A normal value is 1000 mL O2 per minute. |
| Arterial oxygen content | Arterial Oxygen Content = Amount of Oxygen in the Blood Cao2 = (1.39 × Hb × Sao2) + (Pao2 × 0.0031) |
| Oxygen consumption | Oxygen Consumption = Cardiac Output × (Arterial O2 Content – Venous O2 Content) V̇o2 = CO × (Cao2 – Cvo2) Alternative equation: V̇o2 = CO × Hb × 1.39 × (Sao2 – Smvo2) The amount of O2 consumed by tissues each minute is equal to the difference in O2 delivered to tissues and the O2 returning from tissues to the heart. A normal value is about 250 mL O2 per minute. Note that this formula ignores the small contribution from dissolved oxygen. |
| Shock index | Shock Index = Heart Rate ÷ Systolic Blood Pressure SI = HR/SBP A normal value is 0.5–0.7. A persistent elevation of the shock index (>1.0) indicates an impaired left ventricular function (as a result of blood loss or cardiac depression) and carries a high mortality rate. |
| Cao2 | Arterial oxygen content |
| CI | Cardiac index (cardiac output/body surface area) |
| CO | Cardiac output |
| Cvo2 | Venous oxygen content |
| CVP | Central venous pressure |
| Do2 | Systemic oxygen delivery |
| DBP | Diastolic blood pressure |
| Hb | Hemoglobin |
| MAP | Mean arterial pressure |
| MODS | Multiorgan dysfunction syndrome |
| Paco2 | Partial pressure of arterial carbon dioxide |
| Pao2 | Partial pressure of arterial oxygen |
| Sao2 | Arterial oxygen saturation |
| Scvo2 | Central venous oxygen saturation from the superior vena cava |
| Smvo2 (Svo2) | Mixed venous oxygen saturation from the pulmonary artery |
| SBP | Systolic blood pressure |
| SI | Shock index |
| SIRS | Systemic inflammatory response syndrome |
| SVR | Systemic vascular resistance |
| V̇ o2 | Systemic oxygen consumption |
Tissue oxygenation is predicated on CO being sufficient enough to deliver oxygenated hemoglobin to the tissues. CO is dependent on the interplay of cardiac inotropy (speed and shortening capacity of myocardium), chronotropy (heart contraction rate), and lusitropy (ability to relax and fill heart chambers). Determinants of inotropy include autonomic input from sympathetic activation, parasympathetic inhibition, circulating catecholamines, and short-lived responses to an increase in afterload (Anrep effect) or heart rate (Bowditch effect). Increases in the inotropic state help to maintain stroke volume at high heart rates.6 Under certain conditions, such as shock states, higher levels of epinephrine will be produced and reinforce adrenergic tone. Epinephrine levels are significantly elevated during induced hemorrhagic shock, but these levels subsequently reduce to almost normal levels after adequate blood pressure is restored.7 Previous studies have also shown that an acidotic milieu, which is common in shock, further compromises ventricular contractile force and blood pressure.8 Chronotropy and lusitropy are both influenced by sympathetic input. Norepinephrine interacts with cardiac β1-receptors, resulting in increased cyclic adenosine monophosphate. This leads to a process of intracellular signaling with an increased chronotropy and sequestration of calcium, leading to myocardial relaxation.6
When compensatory mechanisms fail to correct the imbalance between tissue supply and demand, anaerobic metabolism occurs and results in the formation of lactic acid. Lactic acid is rapidly buffered, resulting in the formation of measured serum lactate. Normal venous lactate levels are less than 2.0 mmol/L. Most cases of lactic acidosis are a result of inadequate oxygen delivery, but lactic acidosis occasionally can develop from an excessively high oxygen demand (e.g., status epilepticus). In other cases, lactic acidosis occurs because of impaired tissue oxygen utilization (e.g., septic shock or the postresuscitation phase of cardiac arrest). Elevated lactate is a marker of impaired oxygen delivery or utilization and correlates with short-term prognosis of critically ill patients in the ED.7
Shock provokes a myriad of autonomic responses, many of which serve to maintain perfusion pressure to vital organs. Stimulation of the carotid baroreceptor stretch reflex activates the sympathetic nervous system triggering (1) arteriolar vasoconstriction, resulting in redistribution of blood flow from the skin, skeletal muscle, kidneys, and splanchnic viscera; (2) an increase in heart rate and contractility that increases CO; (3) constriction of venous capacitance vessels, which augments venous return; (4) release of the vasoactive hormones epinephrine, norepinephrine, dopamine, and cortisol to increase arteriolar and venous tone; and (5) release of antidiuretic hormone and activation of the renin-angiotensin axis to enhance water and sodium conservation to maintain intravascular volume.8
These compensatory mechanisms attempt to maintain Do2 to the most critical organs (heart and brain), but blood flow to other organs, such as the kidneys and GI tract, may be compromised. The cellular response to decreased Do2 (adenosine triphosphate depletion) leads to ion-pump dysfunction, influx of sodium, efflux of potassium, and reduction in membrane resting potential. As shock progresses, the loss of cellular integrity and the breakdown in cellular homeostasis result in cellular death. These pathologic events give rise to a cascade of metabolic features including hyperkalemia, hyponatremia, azotemia, hyper- or hypoglycemia, and lactic acidosis.
In the early phases of shock, these physiologic changes may produce a clinical syndrome called the systemic inflammatory response syndrome or SIRS (Table 12-3).
Two or more of the following features are required to make a diagnosis of SIRS: Temperature >38°C (100.4°F) or <36°C (96.8°F) Heart rate >90 beats/min Respiratory rate >20 breaths/min (or carbon dioxide tension <32 mm Hg) WBC count >12.0 × 109/L, <4.0 × 109/L, or >10% immature forms or bands |
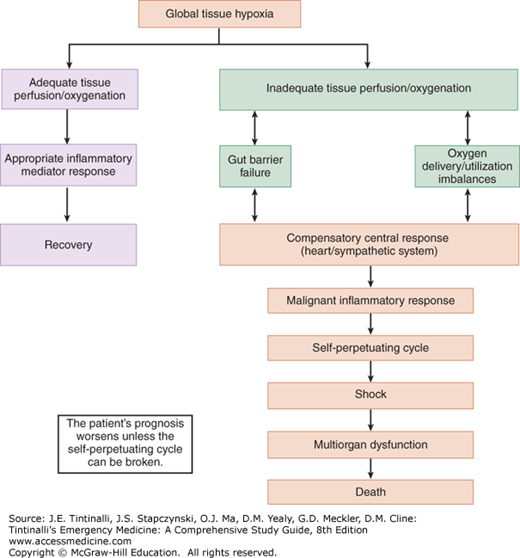
As systemic inflammatory response syndrome progresses, shock ensues, followed by the multiorgan dysfunction syndrome, which is manifested by renal failure, respiratory failure, myocardial depression, liver failure, and then disseminated intravascular coagulation. The fulminant progression from systemic inflammatory response syndrome to multiorgan dysfunction syndrome is determined by the balance of anti-inflammatory and proinflammatory mediators and the level of inadequate tissue perfusion (Figure 12-1).
CATEGORIES OF SHOCK
The four categories of shock can be described in terms of their respective physiologic changes and common causes, recognizing that overlap is common (Table 12-4). Hypovolemic shock occurs when decreased intravascular fluid or decreased blood volume causes decreased preload, stroke volume, and CO. Severe blood loss (hemorrhage) can cause decreased myocardial oxygenation, which decreases contractility and CO. This action may lead to an autonomic increase in the SVR. Hypovolemic shock can also occur due to volume loss from other etiologies. In cardiogenic shock, the left ventricle fails to deliver oxygenated blood to peripheral tissues due to variances in contractility, as well as preload and afterload. Myocardial infarction is the most common cause of cardiogenic shock. Dysrhythmias are another common cause because they can lead to a decreased CO. Bradyarrhythmias result in low CO, and tachyarrhythmias can result in decreased preload and stroke volume. Obstructive shock is due to a decrease in venous return or cardiac compliance due to an increased left ventricular outflow obstruction or marked preload decrease. Cardiac tamponade and tension pneumothorax are common causes. In distributive shock, there is relative intravascular volume depletion due to marked systemic vasodilatation. This is most commonly seen in septic shock. Compensatory responses to decreased SVR may include increased CO (increased contractility and heart rate) and tachycardia. The concurrent decreased SVR results in a decreased preload and may hinder CO overall. In sepsis, up to 40% of patients may have a transient cardiomyopathy characterized by decreased contractility and increased mortality.3,4 Anaphylaxis, adrenal insufficiency and neurogenic shock are additional causes of distributive shock.
| Type | Hemodynamic Changes | Etiologies |
|---|---|---|
| Hypovolemic | Decreased preload, increased SVR, decreased CO | Hemorrhage, capillary leak, GI losses, burns |
| Cardiogenic | Increased preload, increased afterload, increased SVR, decreased CO | MI, dysrhythmias, heart failure, valvular disease |
| Obstructive | Decreased preload, increased SVR, decreased CO | PE, pericardial tamponade, tension PTX |
| Distributive | Decreased preload, increased SVR, mixed CO | Sepsis, neurogenic shock, anaphylaxis |
CLINICAL FEATURES
While the clinical presentation of a patient in shock and the underlying cause may be quite apparent (e.g., acute myocardial infarction, anaphylaxis, or hemorrhage), it may be difficult to obtain a history from patients in shock. Assistance with medical history from EMS, family, or other sources may help determine the cause of shock, especially if the patient has comorbidities. Some patients in shock may have few symptoms other than generalized weakness, lethargy, or altered mental status. If the patient is unresponsive, consider trauma as a primary or secondary complication.
Shock is usually associated with systemic arterial hypotension—systolic blood pressure <90 mm Hg. Blood pressure is the product of flow and resistance (MAP = CO × SVR). Blood pressure may not drop if there is an increase in peripheral vascular resistance in the presence of decreased CO with inadequate tissue hypoperfusion, making blood pressure an insensitive marker for global tissue hypoperfusion. Shock may occur with a normal blood pressure, and hypotension may occur without shock. No single vital sign is diagnostic of shock, and blood pressure is particularly insensitive in the presence of peripheral vascular disease, tachycardia with a small pulse pressure, or cardiac dysrhythmias. Composite physical findings are useful in the assessment of shock (Table 12-5).
| Temperature | Hyperthermia or hypothermia may be present. Endogenous hypothermia (hypometabolic shock) must be distinguished from exogenous environmental hypothermia. |
| Heart rate | Usually elevated; however, paradoxical bradycardia can be seen in shock states due to hypoglycemia, β-blocker use, and preexisting cardiac disease. |
| Systolic blood pressure | May actually increase slightly when cardiac contractility increases in early shock and then fall as shock advances. |
| Diastolic blood pressure | Correlates with arteriolar vasoconstriction and may rise early in shock and then fall when cardiovascular compensation fails. |
| Pulse pressure | Increases early in shock and decreases before systolic pressure begins to drop. |
| Mean arterial blood pressure | Often low, <65 mm Hg. |
| CNS | Acute delirium or brain failure, restlessness, disorientation, confusion, and coma secondary to a decrease in cerebral perfusion pressure |
| Skin/capillary refill | Pallor, pale, dusky, clammy, cyanosis, sweating, altered temperature, and increased capillary refill time of >2–3 s. |
| Cardiovascular | Neck vein distention or flattening depending on the type of shock. Tachycardia and arrhythmias. An S3 may result from high-output states. Decreased coronary perfusion pressures can lead to ischemia, decreased ventricular compliance, increased left ventricular diastolic pressure, and pulmonary edema. |
| Respiratory |