119 Antimicrobials in Chemotherapy Strategy
Infections are frequently suspected or documented in critically ill patients. Patients are often admitted to the intensive care unit (ICU) for treatment of community-acquired or hospital-acquired infections, whereas many other patients require treatment for nosocomial infections acquired during their ICU stay. Although patients in ICUs represent only 8% to 15% of hospital admissions in the United States,1 these patients suffer a disproportionately high rate of infectious complications and are exposed to very high rates of antimicrobial use.1–3 The importance of antimicrobial drugs in the modern management of critically ill patients with a variety of bacterial, fungal, and viral infections can scarcely be understated. However, despite the availability of improved diagnostic techniques and a wide variety of potent, highly effective antimicrobials, the prevention and appropriate treatment of infections in ICU patients remain a formidable challenge to the clinician.
 Antimicrobial Resistance in the ICU
Antimicrobial Resistance in the ICU
The continuing emergence of antimicrobial resistance in ICUs is a major factor in the appropriate selection and use of antimicrobials in the critical care setting. It has been estimated that 50% to 60% of all nosocomial infections occurring each year in the United States are caused by antimicrobial-resistant strains of bacteria.3,4 The overall incidence of infections due to antibiotic-resistant pathogens, changes in the epidemiology of infections caused by specific pathogens, and increasing resistance to even the most potent broad-spectrum agents make the selection of appropriate antimicrobial therapy extremely challenging in many institutions.1–4 The difficulties in selecting antimicrobial therapy are particularly acute in ICUs because of the higher prevalence of antimicrobial resistance in these areas compared with other non-ICU settings.1,5–8
A number of factors are associated with high rates of antimicrobial resistance in the ICU. Chief among these is the heavy use of antimicrobial agents in critically ill patients. A number of studies have identified a close association between antimicrobial use and the subsequent development of antibiotic resistance.9–19 Whereas use of antibiotics is associated with the emergence of resistance during therapy, previous exposure to antibiotics is also a well-established risk factor for antimicrobial resistance.1–38 The higher severity of illness found among ICU patients is also related to several other risk factors for antimicrobial resistance, including the presence of invasive devices such as endotracheal tubes and intravascular and urinary catheters, prolonged length of hospital stay, immune suppression, and malnutrition.1,2,4,5,8–19 The increasing prevalence of antimicrobial-resistant pathogens among residents in long-term care facilities is also an increasingly important source for resistant bacteria in ICUs.1–5,8,20 Finally, antimicrobial-resistant pathogens are easily cross-transmitted among patients in ICUs, owing to poor adherence of hospital personnel to appropriate infection prevention techniques, contamination of equipment, and frequent overcrowding of patients.1–58 All of these various factors combine to make ICUs the epicenter of antimicrobial resistance in hospitalized patients.1,5
Increased antimicrobial resistance has been observed among both gram-positive and gram-negative bacteria as well as among certain fungi, particularly Candida species. Table 119-1 summarizes important trends in increasing resistance in the United States among selected pathogens and drug classes.1,3,4,21 Much of the changing epidemiology of infection in the ICU has centered around the emergence of gram-positive organisms as predominant pathogens in the critically ill patient. Surveillance programs such as the National Healthcare Safety Network [NHSN], which incorporates the former National Nosocomial Infection Surveillance (NNIS) System sponsored by the Centers for Disease Control and Prevention, have repeatedly documented impressive increases in antimicrobial resistance among pathogens such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant Streptococcus pneumoniae.1–320
TABLE 119-1 Trends in Antimicrobial Resistance Among Selected Nosocomial Pathogens from ICU Patients in the United States, 1998-2002 and 2006-2007
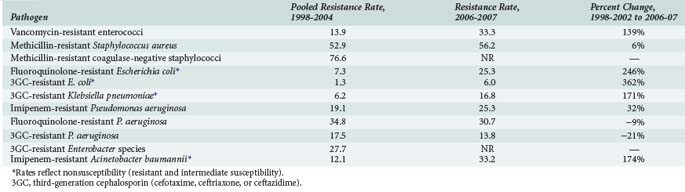
Rates of MRSA and methicillin-resistant coagulase-negative staphylococci have continued to steadily increase over the past decade and are most commonly associated with central catheter-associated bloodstream and wound infections,1–3,5,7,8 whereas MRSA has also been increasingly documented as a frequent pathogen in ventilator-associated pneumonias as well as skin/soft tissue and other infections.1,21–23 Although MRSA has been traditionally regarded as a hospital-acquired pathogen, this bacteria has also emerged as a common cause of community-acquired infections1,24,25; approximately 30% to 40% of all MRSA isolates found in hospitals are now actually community acquired.26 The increase in methicillin resistance among staphylococci has led to a heavy reliance on vancomycin as a drug of choice for infections due to these pathogens and is perhaps related to the dramatic increase in the number of infections caused by VRE among ICU patients. High-level penicillin resistance among S. pneumoniae is approximately 20% to 30% in most geographic areas.27–29 Additionally, penicillin-resistant pneumococci tend to be multidrug resistant; 25% to 30% of S. pneumoniae have decreased susceptibility to macrolide antibiotics, and rates of resistance to several other drug classes including sulfonamides, tetracyclines, and cephalosporins have also increased.27–29 Although the prevalence of fluoroquinolone resistance among S. pneumoniae is still very low (<1%),27–29 there is still significant concern regarding excessive use of fluoroquinolones and the potential for significant resistance in the future.27–30
Antimicrobial resistance continues to be a problem of major importance among gram-negative bacilli. Of particular concern is the rapid spread of resistance mediated by extended-spectrum β-lactamases (ESBLs) among organisms such as Escherichia coli and Klebsiella pneumoniae. Organisms that produce ESBLs are usually resistant to multiple antimicrobials, including third- (e.g., ceftriaxone, ceftazidime) and fourth-generation (e.g., cefepime) cephalosporins and aztreonam, and are also associated with high rates of resistance to aminoglycosides and fluoroquinolones.31–34 The increase in ESBL-mediated resistance is reflected in rates of E. coli and K. pneumoniae resistance to third-generation cephalosporins, as shown in Table 119-1. Antimicrobial resistance among Pseudomonas aeruginosa is also alarming in that nearly all major drug classes are currently being affected; nearly 10% of P. aeruginosa isolates are now resistant to multiple drug classes, including cephalosporins, carbapenems, aminoglycosides, and/or fluoroquinolones.* Multidrug resistance is also very common (approximately 30% of isolates) among strains of Acinetobacter baumanii.4,35 Fluoroquinolone resistance is being increasingly reported among organisms such as E. coli that were previously considered to be extremely susceptible to this class of drugs.1–436 Antimicrobial resistance among gram-negative organisms such as P. aeruginosa has been of great concern in the ICU setting for many years, but increasing resistance among previously susceptible organisms and the involvement of multiple drug classes clearly indicates that the problem continues to grow worse. An additional troubling development in recent years is the detection of K. pneumoniae carbapenemase (KPC) enzymes which, as the name implies, confer resistance to a broad range of β-lactam-type antibiotics including the carbapenems.37 The increase in ESBL-producing strains and other multidrug-resistant pathogens has led to a heavy reliance on the carbapenems for treatment of gram-negative infections. Although KPC-producing strains are still relatively uncommon, their rapid spread through many geographic areas has led to serious concerns regarding the loss of carbapenems as reliable agents for empirical or “definitive” (i.e., based on culture and susceptibility information) treatment of many infections in ICU patients.37
Candida albicans is now the fourth most common pathogen associated with nosocomial infections in critically ill patients in the United States. While C. albicans is associated with approximately 7% of all nosocomial infections, it is the second most common cause of nosocomial urinary tract infections (15% of infections), the third most common cause of central line–associated bloodstream infections (6% of infections), and fourth most common cause of all nosocomial bloodstream infections.1,2 Resistance to antifungal agents among Candida species is now a significant problem in many hospitals, with fluconazole resistance being reported in up to 10% of C. albicans isolates from bloodstream infections.38–40 Because susceptibility testing for Candida species is not routinely performed in most hospitals, the true scope of resistance among C. albicans and other strains is not well characterized and may in fact be higher than currently assumed. It is well documented, however, that the relative frequency of fungal infections with Candida glabrata, Candida krusei, and other strains with decreased susceptibility to azole antifungals is increasing among certain populations, such as the critically ill and patients with hematologic malignancies.38–40 The increased proportion of non-albicans strains of Candida is particularly problematic because it has often led to the use of non-azole type agents such as the echinocandins for empirical therapy of patients at high risk for Candida infections.38–40
Infections caused by antimicrobial-resistant bacteria have been demonstrated to be associated with higher mortality rates, longer length of ICU and hospital stays, and higher medical costs.41–44 Antimicrobial-resistant strains of bacteria have been demonstrated to express virulence factors that may be different from those expressed by antimicrobial-susceptible strains; this may explain some of the increased mortality associated with these infections.6,7,37,44 However, increased mortality associated with infections caused by resistant bacteria may also be explained by the increased likelihood that patients will receive inadequate antimicrobial treatment. Inadequate antimicrobial therapy, defined as the use of drugs with poor in vitro activity against the infecting pathogen and/or improper dosing of drugs, has been demonstrated in numerous studies to be significantly associated with increased mortality and other measures of poor patient outcomes.45–54 Treatment with inadequate antimicrobial therapy is particularly problematic during the initial empirical treatment of infections when specific pathogens and antibiotic susceptibility information are not yet known.45,47,51,53,54 It is logical to assume that selection of adequate empirical therapy becomes more difficult as the organisms become more resistant to antimicrobial therapy, and it has in fact been demonstrated in clinical studies that most inadequate treatment of nosocomial infections in the ICU is related to the presence of pathogens that are resistant to the selected antibiotics.46,48,51–53 Furthermore, it has been shown in patients with nosocomial pneumonia that changing to more appropriate antibiotics when culture and susceptibility results became available (typically 48–72 hours after initiating therapy) did not significantly lower mortality rates compared with patients who received inadequate antibiotics for the entire duration of therapy.45 The importance of antimicrobial resistance in terms of antimicrobial selection and patient outcomes is thus difficult to overstate.
 Strategies to Reduce Antimicrobial Resistance
Strategies to Reduce Antimicrobial Resistance
Various strategies have been recommended to decrease problems of resistance through improved use of antimicrobials. These strategies include the use of antimicrobial protocols and guidelines, hospital formulary-based antimicrobial restrictions, scheduled antimicrobial rotation or “cycling,” improved techniques for detection and/or diagnosis of infections, improved dosing of antimicrobials based on pharmacokinetic and pharmacodynamic concepts, use of combination antimicrobial therapy, decreased duration of antimicrobial therapy, and early involvement of infectious diseases specialists in the management of infected patients.55 All of these various strategies fall within the realm of “antimicrobial stewardship,” a process for collectively improving the overall use of antimicrobials through many different means.56
Among these various strategies, the roles of antimicrobial restrictions and antimicrobial cycling are two particularly controversial issues. Hospital formulary–driven restriction of specific drugs or drug classes is a common method of controlling antimicrobial use within an institution. Formulary-based restrictions have historically been used to control drug costs; they may also reduce rates of adverse effects of high-risk agents.56 Antimicrobial restrictions are also used in an attempt to either decrease overall emergence of antimicrobial resistance within an institution or to control acute outbreaks of resistance affecting specific drugs and pathogens.56–59 The effectiveness of antimicrobial restrictions in reducing overall levels of resistance has not been consistently demonstrated. Indeed, it can be argued that antimicrobial restrictions cause intense selective pressure from a small number of agents and may actually promote the emergence of resistance rather than preventing it.60 Antibiotic restrictions that are instituted in response to specific outbreaks of antibiotic-resistant infections, together with appropriate infection control measures, have been shown to successfully manage specific resistance problems.56–59 However, it has also been shown that restriction of a drug in response to a resistance issue may in turn cause other resistance problems affecting other drugs.60 This phenomenon is sometimes referred to as “squeezing the balloon” because the enforcement of antimicrobial restrictions leads to new selective pressures that may effectively solve the original problem but cause the development of new resistance issues.61 A classic example involved restriction of ceftazidime and increased use of imipenem in response to an outbreak of ceftazidime-resistant K. pneumoniae. Although ceftazidime resistance among K. pneumoniae isolates was effectively decreased by 44%, the rates of imipenem-resistant P. aeruginosa significantly increased by 69%.60 Although antimicrobial restrictions may be effective in reducing drug costs and limiting specific outbreaks of resistant infections, the emphasis must clearly be on appropriate and rational drug use rather than relying on such restrictions to overcome resistance problems.
Antibiotic cycling, in which a specific drug or an entire antibiotic class is periodically withdrawn from clinical use and replaced with a different drug or class, has been investigated as a means of decreasing resistance by limiting narrow selective pressures and exposing organisms to a wide variety of different antimicrobials over time.62–65 Although initial studies were promising and demonstrated reduced antimicrobial resistance as well as decreased incidence of certain nosocomial infections and reduced patient mortality,62–65 these studies have not been consistent in the overall effectiveness of the antibiotic cycling strategy. In addition, a number of important questions concerning antibiotic cycling have not been adequately addressed by previous studies. These questions include which specific agents or classes are most appropriate to cycle, whether agents or classes of drugs should be cycled in a specific order, how often to change drugs within the scheduled cycle, and whether the potential effectiveness of antimicrobial cycling is maintained over long periods of time.62–65 Further research is clearly needed to answer these and other relevant questions, and cycling is currently not widely accepted as an effective means of improving infection-related patient outcomes and reducing resistance.
 Principles of Appropriate Antimicrobial Use
Principles of Appropriate Antimicrobial Use
Whereas many of the issues regarding antimicrobial use in critically ill patients are currently centered on issues related to antimicrobial resistance, adherence to basic principles of appropriate drug use is still crucial in overall optimization of drug therapy (Table 119-2).
TABLE 119-2 Basic Principles of Appropriate Antimicrobial Use in Critically Ill Patients
Diagnostic Issues
Establishing a definitive diagnosis of infection is paramount to the appropriate selection and use of antimicrobials. Once infection is suspected in the ICU patient, a comprehensive workup must be performed to identify the site of infection. The microbial causes of various ICU infections are reasonably predictable once the actual site of infection is known; appropriate drug selection thus properly begins with identification of a known or suspected site of infection. Unfortunately, the site of infection is often unable to be identified with any certainty; studies in septic patients have shown that no source of infection is identified in up to 30% to 40% of patients.53,66 Modern ICU practitioners have access to a wide range of invasive and noninvasive diagnostic techniques, and these should be employed when appropriate. However, the institution of antimicrobial therapy should not be unnecessarily delayed for the sake of performing exhaustive diagnostic tests. Although not yet in common use, polymerase chain reaction (PCR) and other molecular-based laboratory methods may offer the potential to improve detection of causative pathogens and facilitate the early initiation of appropriate antimicrobial therapy.67,68
Gram stain of appropriate specimens from potential sites of infection should also be utilized to help determine appropriate empirical or antimicrobial therapy. Although the yield of useful information from Gram stains is usually not high in critically ill patients, performing this test is nevertheless of value for those patients in whom causative pathogens are identified.45,67,68 Gram stains from specimens obtained from certain sites such as the respiratory tract and wounds should be interpreted with caution, owing to high rates of colonization with nonpathogenic organisms, particularly in patients who have already been hospitalized for several days. Studies have clearly demonstrated the high frequency and rapid time course of microbial colonization of ICU patients.69–71 Classic studies demonstrated that rates of colonization of the oropharynx and bronchi of critically ill patients with gram-negative organisms reached 45% and 65% within 5 days after ICU admission, respectively, and over 90% at both sites by day 10.72 These patients also become highly colonized with gram-positive cocci and particularly yeast soon after ICU admission.
Clinicians must keep in mind that there are numerous sources of fever in critically ill patients that are not associated with infection (Table 119-3). The occurrence of new fever in an ICU patient should prompt a thorough evaluation of noninfectious sources for the fever before initiation of antimicrobial therapy. Patients who have been started on antimicrobial therapy and have persistent fever despite the resolution of other signs and symptoms of infection should also be evaluated for noninfectious sources of fever.
TABLE 119-3 Noninfectious Sources of Fever in Critically III Patients
| Hemorrhage |
| Inflammatory Conditions |
| Medications |
| Metabolic Conditions |
| Neoplasms |
| Thromboembolism |
Selection of Empirical Drug Therapy
Initial selection of adequate drug therapy is of vital importance in optimizing outcomes of antimicrobial use in critically ill patients. Selection of inadequate therapy has been demonstrated in numerous clinical studies to be associated with increased patient mortality,45–54 and the risk of inadequate therapy is often directly related to rates of antimicrobial resistance in certain pathogens.46,48,51–53 A number of factors are therefore important to consider when choosing initial empirical therapy. These considerations should include suspected site(s) of infection and corresponding potential pathogens, rates of resistance of these pathogens to potentially used drugs, a patient’s prior exposure to antimicrobial therapy that may potentially increase the likelihood of antimicrobial resistance, and the results of any pertinent prior diagnostic tests. A reasonable understanding of the pharmacology, pharmacokinetics, pharmacodynamics, potential toxicities, potential drug interactions, and appropriate dosing of individual antimicrobials is also important in the selection of a specific agent once the type of drug to be used has been decided on. These drug-specific considerations are discussed in more detail later in this chapter. In general, empirical antimicrobial regimens for critically ill patients should be aggressive, that is, sufficiently broad spectrum in pharmacologic activity to cover the most likely (rather than all possible) pathogens, initiated promptly, and given in relatively high doses when the presence of any significant renal or hepatic dysfunction is considered.
Clinicians should be familiar with patterns and rates of resistance of key pathogens involved in both community-acquired and nosocomial infections. Resistance rates for pathogens occurring in community-acquired infections may be very different from those same types of pathogens causing nosocomial infections.1 For example, E. coli causing community-acquired urinary tract infections may have a rate of resistance to ciprofloxacin of 1% to 2%, whereas E. coli associated with nosocomial urinary tract infections may display resistance to ciprofloxacin in greater than 10% to 15% of strains.73,74 Likewise, S. aureus associated with community-acquired infections is usually susceptible to methicillin, whereas the rate of MRSA is now 60% to 70% in many hospitals in the United States.1,3 Information concerning rates of antimicrobial resistance in the outside community is often not as readily available as information concerning institutional susceptibilities, but ICU practitioners should nevertheless be familiar with resistance rates in both settings in order to choose appropriate antibiotics. Although antibiograms summarizing drug susceptibilities of key pathogens are available in most institutions, clinicians should recognize that published susceptibilities often do not differentiate between ICU and non-ICU isolates. It is well recognized that resistance rates are often much higher among isolates obtained from patients in ICUs where antimicrobial use is heaviest and more risk factors for resistance (e.g., higher severity of illness, invasive devices, immune suppression) are present.1,2,4,5,8–19 It is also known that susceptibilities often differ markedly among different types of ICUs (e.g., medical, surgical, burn, trauma) owing to patients with varying risk factors and potential differences in the types and amounts of antimicrobials used in each of these areas.2,3 When such information is available, ICU practitioners must be aware of any important differences between unit-specific drug susceptibilities and resistance rates for the institution as a whole. Appropriate use of such information can lead to more effective drug selection and enhance the provision of adequate drug therapy.75
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree




