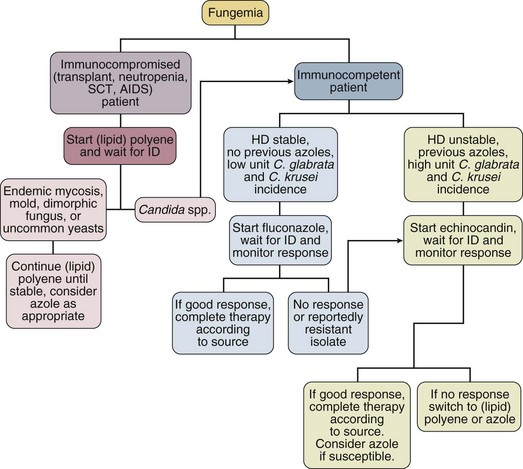
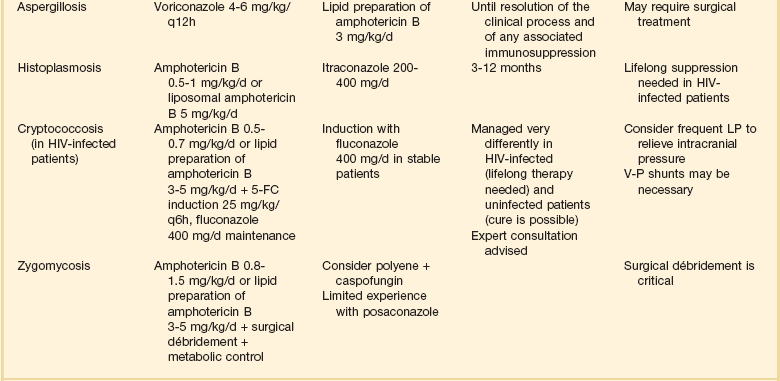
52 SPECIFIC INDICATIONS AND USES FOR ANTIFUNGAL THERAPY AREAS OF CONTROVERSY IN ANTIFUNGAL THERAPY Empirical Antifungal Therapy for the Febrile ICU Patient Antifungal Prophylaxis in the ICU Duration of Therapy and Accumulated Dosing SPECIFIC INDICATIONS AND USES FOR ANTIVIRAL THERAPY This chapter will focus on systemic antifungal therapy as it is relevant to the critical care setting. Topical therapy of candidiasis and therapy of other systemic fungal infections will be omitted or only briefly reviewed. The interested reader is referred to the Infectious Diseases Society of America (IDSA) guidelines on the topic.1 Polyenes act by binding to ergosterol in the fungal cytoplasmic membrane, causing ionic leakage and osmotic instability.2 Additionally, they cause oxidation of the cytoplasmic membrane.2 Polyenes are fungicidal in most settings and the most prominent members of the family are amphotericin B and nystatin. Both drugs have important toxic effects that often limit their use in patients with organ/system failure. Newer formulations of the drugs have been developed to reduce this limitation. Amphotericin B is produced by Streptomyces nodosus and is one of the oldest and most widely used antifungal agents. There is much experience with its use in mycoses and hosts, and it is usually the comparison standard for new therapies.3 Because of significant acute and chronic toxicities, there is an extensive anecdotal literature describing ways to ameliorate these toxicities, as well as limitations of all these strategies.4 The advent of lipid-based formulations of amphotericin B has enhanced our ability to limit toxicities and provide adequate courses of therapy.5 Amphotericin B has in vitro and in vivo activity against most isolates of Candida spp., Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, Mucorales, Coccidioides immitis, Paracoccidioides brasiliensis, Aspergillus spp., Fusarium spp., and Sporothrix schenckii.6 The activity of amphotericin B is so broad that it is almost easier to think of this in terms of the few species that are consistently less susceptible or actually resistant to amphotericin B: Aspergillus terreus,7 Trichosporon spp.,8 and Pseudallescheria boydii (Scedosporium prolificans).9 One species of Candida, C. lusitaniae, readily becomes resistant to the polyenes.10 Resistance among isolates of Candida is otherwise rare.11,12 Amphotericin B is hydrophobic and is combined with deoxycholate to permit intravenous (IV) administration in an aqueous solvent. Once in the bloodstream, it dissociates and binds to plasma proteins and lipoproteins. It is stored in the liver and other organs and slowly eliminated. Drug metabolism is complex and not affected by renal or liver failure.3 Hemodialysis and peritoneal dialysis do not remove the drug. Measuring drug levels is possible13 but not of obvious clinical relevance. Cerebrospinal fluid (CSF) penetration is poor. Although amphotericin B is primarily used in IV infusion, it can also be used topically for localized gastrointestinal (GI) or urinary infections or instilled directly to treat central nervous system (CNS) infections. The most common toxicity is the systemic reaction associated with IV infusion, thought to be caused by release of inflammatory mediators from monocytes and macrophages and producing fever, hypotension, and on occasion severe dyspnea. Two forms of renal toxicity are seen. First, a very acute form of renal dysfunction appears to be related to amphotericin B–induced renal arteriolar constriction.14 Second, cumulative dose-dependent tubular damage is almost invariably seen if significant doses are given. The tubular injury is characterized by potassium/magnesium wasting and azotemia. It is generally reversible when the drug is stopped, but it is also particularly aggravated if the patient is volume depleted or given other nephrotoxic drugs. The renal injury also reduces erythropoietin production15 and thus causes a mild anemia during chronic therapy (the hematocrit will typically fall to about 30%). Finally, amphotericin B may precipitate cardiac arrhythmias, especially if the patient is already hypokalemic and hypomagnesemic.16–19 The deoxycholate formulation of amphotericin B is given intravenously at 0.5 to 1.0 mg/kg/day, but it can also be administered every other day by doubling the dose. To avoid producing a precipitate, it must be diluted in an electrolyte-free solution at no more than 0.1 mg/mL. It should be administered over no less than 1 hour and most authorities prefer a 2- to 3-hour infusion.20 Because the occasional patient reacts violently to the drug, an initial test dose of 1 mg of the drug may be given prior to infusing the entire first dose. Premedication with acetaminophen, diphenhydramine, and steroids may be used if the patient develops reactions to the infusion. Volume loading appears to reduce nephrotoxicity, and many authorities give (if possible) 500 to 1000 mL normal saline just prior to each dose of amphotericin B. Administration via a central line is advisable because amphotericin B given peripherally often produces phlebitis. Treatment goals have been traditionally and arbitrarily cumulative (such as a total of 1 or 2 g), but a time-based approached is increasingly used for some diseases (e.g., for candidemia, in which 2 weeks of therapy at 0.6-0.7 mg/kg after the last positive blood culture has been shown to produce a late relapse rate of about 1%).21 Although the importance of therapy in the typical patient is unclear,22,23 candiduria is sometimes treated with amphotericin B bladder washes. A typical dose is 50 mg of amphotericin B diluted in 1000 mL of water and irrigated over 24 hours. A small number of intracranial fungal infections benefit from intrathecal dosing at 0.1 to 0.5 mg three times per week, but expert advice should be sought if this therapy is considered. There are three lipid-based formulations of the drug: amphotericin B colloidal dispersion (ABCD, marketed as Amphotec and Amphocil), amphotericin B lipid complex (ABLC, marketed as Abelcet), and liposomal amphotericin (L-AmB, marketed as AmBisome). The names of these compounds are often confusing. Only one of the drugs (L-AmB) is a true liposome. However, it is not uncommon for physicians to refer to therapy with these compounds in general as therapy with “liposomal amphotericin B.” The preferred terminology is “lipid-associated formulation of amphotericin B” (LFAB).5 When speaking of specific compounds, we find that use of the names ABCD, ABLC, and AmBisome minimizes confusion. All three LFABs have comparable efficacy among themselves and when compared to regular amphotericin B, but significantly less nephrotoxicity.5,24 They are also thought to be concentrated and distributed in the reticuloendothelial system, theoretically achieving higher tissue dose delivery and concentration. The lipid carrier does, however, dramatically change the pharmacology and delivery of these compounds, and higher doses than are typical for amphotericin B are both safe and necessary for optimal activity: The licensed dosages are 5 mg/kg/day (ABLC), 3 to 6 mg/kg/day (ABCD), and 3 to 5 mg/kg/day (AmBisome). The optimal dosage of these compounds is unclear and the agents appear generally equipotent. Dosages of approximately 3 mg/kg/day would appear suitable for treatment of most serious Candida infections. Doses of at least 5 mg/kg/day are used for mold infections, with some authors recommending even higher doses.25–27 However, a recent study showed no advantage to using 10 mg/kg/day over 3 mg/kg/day for invasive aspergillosis and other invasive mold infections.28 All three LFABs appear active against the same range of fungal infections that can be treated with amphotericin B. The compounds do differ in their relative toxicities.24 ABCD appears to have significant administration-related toxicity and is not often used. AmBisome and ABLC are both well tolerated, but AmBisome has been associated with somewhat less nephrotoxicity in some patient settings.29 Some patients will tolerate one formulation better than another.30,31 Because of the cost of the LFABs, there has been great interest in the concept of making a pseudo-LFAB by suspending amphotericin B deoxycholate in commercially available lipid emulsions.32–34 This practice does not, however, consistently reduce toxicity.35 This may be due to preparation-dependent precipitation of the amphotericin B noted by some36,37 but not all38 authors. Most authorities have concluded that further work with this approach should be undertaken as part of a controlled clinical trial that addresses these issues.39 A recent meta-analysis on the use of these formulations has been published, but because of heterogeneity, the results are inconclusive.40 The cost of the LFABs is significant, but the counterbalancing reduction in nephrotoxicity is also valuable and should be considered when choosing an LFAB versus a conventional amphotericin B.41 A recent study of the impact of nephrotoxicity of amphotericin B deoxycholate in patients with invasive aspergillosis suggested that this patient population suffered significant morbidity due to the amphotericin B itself.42 Use of amphotericin B deoxycholate produces significant nephrotoxicity in about 30% of patients, increasing hospital length of stay and hospitalization costs by nearly $30,000.43,44 Nevertheless, the context of amphotericin B–induced toxicity should also be considered. A rise of the creatinine to 3 mg/dL in an otherwise well patient who is being treated with amphotericin B for (say) osteoarticular sporotrichosis may be clinically imperceptible owing to the fact that the patient has no other acute medical problems. On the other hand, a rise in creatinine from 1 mg/dL to 2 mg/dL may be disastrous in a surgical patient who is also suffering from nosocomial pneumonia and cardiac insufficiency. No firm guidelines in this area have yet to emerge. We currently believe that an LFAB is appropriate for patients who have failed amphotericin B deoxycholate (FDA [Food and Drug Administration] indication), or who are intolerant of amphotericin B deoxycholate (FDA indication), or who are highly likely to be intolerant (no FDA indication). We define intolerance broadly: a creatinine clearance (CrCl) less than 50% of the normal for the patient’s age or a fall in CrCl with therapy. Predicting intolerance is difficult, but such factors as concomitant use of highly nephrotoxic agents (e.g., an aminoglycoside) or underlying primary/intrinsic renal disease (e.g., diabetes mellitus) associated renal dysfunction should be considered. Flucytosine (5-FC) is the fluorine analog of cytosine.45 It was originally synthesized as an antineoplastic agent, but poor antitumor activity and discovery of its antifungal properties led to its further development as an antifungal agent. It acts by deamination to 5-fluorouracil and then conversion to a noncompetitive inhibitor of thymidylate synthase that interferes with fungal DNA and RNA synthesis. It has been demonstrated effective in cryptococcosis, candidiasis, and chromomycosis, being the drug of choice for the latter infection. Drug resistance in vivo develops quickly, so the standard practice is to combine it with another agent.46,47 5-FC is water soluble and has high bioavailability. Protein binding is negligible and approximately 90% is excreted in urine. CSF penetration is good, and both hemodialysis and peritoneal dialysis remove it. It is teratogenic in rats and therefore contraindicated during pregnancy. Adverse effects include rash, diarrhea, and hepatic dysfunction. High blood levels (>100 µg/mL) are associated with profound leukopenia and thrombocytopenia.48 This has prompted the recommendation of monitoring drug levels, renal/liver function, and blood counts in patients receiving 5-FC. Dosage of 150 mg/kg/day divided in four doses has usually been suggested, but recent in vivo49 and human experience50 suggests that dosage of 100 mg/kg/day (again, divided into four doses) may be as effective and better tolerated. Renal failure requires dosage adjustment to half the dose if CrCl is 25 to 50 mL/minute and a quarter dose if it falls below 25 mL/minute. Patients on hemodialysis should be given the latter dose after dialysis. Combination with amphotericin requires constant monitoring of toxicity parameters and dose adjustment. The target blood level is approximately 50 µg/mL. Most experts would discontinue use of this agent if blood counts start dropping, regardless of the blood levels. The introduction of this class of drugs was a major advance in antifungal therapy, because they offer both IV and oral formulations for the treatment of systemic mycosis. Their widespread use has also prompted the emergence of resistance. Azoles act by blocking the activity of lanosterol demethylase, a cytochrome enzyme in both fungal and mammalian cells. Fungal cell membrane synthesis of ergosterol is inhibited and other sterol intermediates are substituted in the membrane, resulting in a nonviable cell. This effect is much slower than that of amphotericin, so these drugs are generally regarded as fungistatic. Because these drugs reduce production of the ergosterol to which polyenes must bind to produce their effect, there is a potential for the azoles and polyenes to appear antagonistic. However, these effects are drug-, organism-, and model-dependent and a range of effects may be seen. This area is complex and has recently been reviewed.51,52 At present, use of such combinations should be avoided outside a clinical trial. All of the azoles have the ability to interfere with mammalian sterol synthesis. This was most notable with ketoconazole, which can produce gynecomastia and adrenal insufficiency.53,54 Subsequent azoles have been selected for lack of such effects. All of the azoles can, however, produce hepatic dysfunction. The most common pattern is that of increased transminases. However, any form of dysfunction may be seen. This can be life-threatening if not recognized. The hepatic dysfunction is reversible upon discontinuation of the offending azole. Another concern with azoles is drug interactions. This becomes particularly important in the critical care setting in which many drugs are being used concomitantly. Table 52.1 summarizes the most important drug interactions that have been reported. The critical interactions generally have to do with drugs cleared by the liver. Some agents (e.g., rifampin and phenobarbital) induce the enzymes that clear the azoles. In other cases, the azole interferes with clearance of another agent (e.g., the azoles predictably increase blood levels of cyclosporine). It is not possible to list all of the known interactions. Consultation with a pharmacy specialist is suggested when using azoles in the setting of polypharmacy, particularly in the critical care setting and when caring for patients with multiple comorbid conditions. Table 52.1 Selected Azole-Drug Interactions* *Generally, azoles tend to increase the levels of other drugs, whereas other drugs tend to decrease the level of the azole. This list is not meant to be comprehensive, and the practitioner should review the full prescribing information. Consultation with a pharmacy specialist is recommended when azoles are going to be used in complex polypharmacy situations. Fluconazole is one of the newer triazoles that has in vitro and in vivo activity mainly against yeast, such as Candida spp. and Cryptococcus neoformans. It also has efficacy against Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis. Unfortunately resistance (mediated by increased production of target enzymes, efflux pumps, and mutations in target enzyme) has become a problem, particularly in Candida albicans (mutation to resistance is seen), C. glabrata (has intrinsically lower susceptibility and may become highly resistant), and C. krusei (intrinsically highly resistant).55,56 Nevertheless, fluconazole is still very active against most strains causing invasive candidiasis, and higher doses can be used for those organisms that are in the “susceptible-dose dependent” range. Fluconazole is water soluble and is available in oral and IV presentations that produce similar blood levels. Bioavailability is excellent and it is well absorbed regardless of the gastric contents. It has a long half-life and can be administered in a single daily dose. It exhibits little binding to serum proteins and is widely distributed in all body fluids. CSF penetration is particularly high, making it particularly useful in the treatment of CNS infections such as cryptococcosis and coccidioidomycosis. Fluconazole is excreted by the kidneys and dosing should be adjusted in proportion to the CrCl. The most common adverse effects are nausea and vomiting. Skin rash is infrequent but can be severe. Hepatitis has also been reported. The usual dose is 400 to 800 mg (or 6-8 mg/kg) per day by mouth or IV, but doses as high as 2 g/day have been tolerated.57 Itraconazole is a triazole antifungal agent with a wider spectrum than fluconazole. It has in vitro and in vivo activity against Candida spp., Aspergillus spp., Histoplasma spp., Blastomyces dermatitidis, Sporothrix schenckii, Trichophyton spp., Cryptococcus neoformans, Coccidioides immitis, and Paracoccidioides brasiliensis.58,59 The resistance issues observed for fluconazole are also an issue with itraconazole.56 Itraconazole was initially available only in a capsule form that has unpredictable bioavailability. Absorption of the capsule formulation is optimized by ingestion with food.60 Two new formulations have recently been introduced. First, there is a solution in cyclodextrin for oral administration that significantly enhances bioavailability and is now preferred over the capsule for producing maximal blood levels.61 Second, an IV formulation (again, in a cyclodextrin carrier) has recently become available. The IV formulation is valuable in that it quickly and reliably produces significant blood levels.62–64 Itraconazole is highly protein bound and has a prolonged half-life. CSF penetration is negligible, so it is not generally used for CNS infections. Itraconazole is metabolized by the liver and dosing does not need to be modified in renal failure. However, the cyclodextrin carrier used in the IV formulation is cleared by the kidneys and its behavior in patients with renal dysfunction is not known. Thus, this formulation of itraconazole should not be used in patients with a CrCl less than 25 mL/hour. Adverse effects are mainly GI, with nausea, vomiting, and abdominal pain occurring in up to 10% of patients. Hepatitis is uncommon but liver enzyme monitoring is recommended. Because itraconazole, and its major metabolite hydroxyitraconazole, are inhibitors of CYP3A4, drug interactions are a major issue and the most common ones are summarized in Table 52.1. Voriconazole is one of the newest triazoles to arrive into the market. It is licensed for the treatment of invasive candidiasis and invasive aspergillosis, as well as for the treatment of Fusarium and Scedosporium infections. It is considered as the treatment of choice for invasive aspergillosis.65 Voriconazole was not inferior to amphotericin B followed by fluconazole in a large clinical trial, but it is unknown if it has any advantages over fluconazole or the echinocandins for treating infections by fluconazole-resistant C. glabrata.66,67 Although it has activity against the endemic mycoses, data are limited at this point, so it is not recommended for routine use in these infections at this time.68,69 A relevant gap in its activity is the class of Zygomycetes (such as Mucor spp., Absidia spp., and Rhizopus spp.). Although there have been multiple reports of breakthrough Zygomycetes infections in patients receiving voriconazole prophylaxis or treatment, a causal relationship has not been established.70,71 Nevertheless, clinicians should be aware that this drug does not have activity against these organisms, and thus it should not be used for empirical therapy of mold infections if Zygomycetes are in the differential diagnosis. Voriconazole is available in oral (tablets and suspension) and IV formulations, with 96% bioavailability. Usual dosing is 4 to 6 mg/kg IV every 12 hours or 200 to 300 mg orally every 12 hours. Voriconazole has nonlinear pharmacokinetics; thus, increasing the dose will not necessarily increase blood levels. Routine blood level measurement to document absorption is now recommended, although blood levels associated with specific efficacy and safety margins have not been established. Voriconazole is metabolized by the human hepatic cytochrome P-450 enzymes, CYP2C19, CYP2C9, and CYP3A4, and has extensive drug interactions with many drugs commonly used in the critical care setting. Consultation with a pharmacy specialist is recommended for patients on multiple drugs. Like itraconazole, the IV formulation is prepared in a cyclodextrin-based formulation, and thus it is not recommended for use in patients with CrCl less than 50 mL/minute. In such patients the oral formulation may be safely used. Adverse events include self-limited visual disturbances and hallucinations, infrequent reports of hepatic insufficiency, and as with the other azoles, rare reports of arrhythmias and QT prolongation. Monitoring of liver enzymes is recommended during voriconazole therapy.72–74 Posaconazole is the newest triazole in the market and although licensed primarily for prophylaxis of fungal infections in high-risk patients,75 it does have promising activity against mold infections, in particular infections by the Zygomycetes and Fusarium.76 It has also shown excellent in vitro and in vivo activity against Candida spp. with demonstrated efficacy in esophageal disease. It is available only in an oral formulation.77,78 The echinocandin antifungal agents represent an entirely new class of antifungal drugs. There are three echinocandins on the market at this time: caspofungin, micafungin, and anidulafungin. These agents are cyclic lipohexapeptides that act via inhibition of glucan synthesis.79 Preclinical studies have shown efficacy against all species of Candida without any evidence for cross-resistance with polyenes or azoles, Aspergillus spp., and selected other fungi.80,81 Although the target enzyme is present in most fungi, the echinocandins are not active against C. neoformans and molds other than Aspergillus. The echinocandins appear to be rapidly fungicidal for Candida spp., but their activity against Aspergillus may be better described as fungistatic.82 Nonetheless, they are quite active in animal models of both candidiasis and aspergillosis.83,84 These drugs have a long half-life, permitting once-daily dosing, and are excreted in the liver. They do not interfere with the cytochrome system and they are not known to be nephrotoxic, having otherwise remarkable safety profiles. At this time, differences between the three drugs appear to be subtle, requiring further study. Caspofungin was the first echinocandin in the market. It has excellent in vitro and in vivo activity against Aspergillus and Candida spp. It has demonstrated efficacy for the treatment of invasive candidiasis, treatment of invasive aspergillosis, and empirical therapy of fungal infections in the setting of febrile neutropenia.85–87 The usual dosing includes loading with 70 mg IV, followed by 50 mg IV every 24 hours. The safety profile is good with mild elevation of liver enzymes. Patients with hepatic insufficiency (Child-Pugh class B or C) require dosage adjustment to 35 mg/kg IV every 24 hours.68,88,89 Drug interactions are infrequent, but it is recommended to monitor cyclosporine levels in patients receiving caspofungin.90 A recent clinical trial showed that higher doses of caspofungin (100 mg/day) are well tolerated but do not necessarily translate into additional clinical benefits.91 Micafungin is approved by the by the FDA for the treatment of esophageal candidiasis, prophylaxis of Candida infections in stem cell transplant patients, and treatment of candidemia. Although micafungin has excellent in vitro and in vivo activity against Aspergillus and Candida spp., clinical data on invasive aspergillosis are limited.92,93 Micafungin has demonstrated efficacy both for the treatment of invasive candidiasis and as a prophylactic agent for fungal infections in allogeneic stem cell transplant recipients.94–96 The prophylactic dose is 50 mg IV every 24 hours, and the therapeutic dose for invasive candidiasis is 100 mg IV every 24 hours. As with the other echinocandins, the most frequent adverse event is mild elevation of liver enzymes. No dosage adjustment is required in the setting of renal insufficiency or moderate hepatic insufficiency. Mild drug interactions have been reported with concomitant use of sirolimus and nifedipine. As with the other echinocandins, anidulafungin has excellent in vitro and in vivo activity against Aspergillus and Candida spp.97 It is currently indicated for the treatment of invasive candidiasis, having demonstrated noninferiority, and perhaps superiority, in a clinical trial versus fluconazole.98 The usual dosing includes a loading dose of 200 mg IV, followed by 100 mg IV every 24 hours. Anidulafungin does not require dosage adjustment for patients with renal or hepatic failure and no significant drug interactions are reported. 97,99,100 Although the precise diagnosis of a fungal infection may be laborious, time-consuming, and delayed, therapy should never be withheld in a critically ill patient with suspected or confirmed fungal infection. Empirical therapy will often be started with amphotericin B or one of its lipid preparations and then tailored according to the final identification of the organism. This section presents generally accepted treatment recommendations for the most commonly encountered fungal infections in the critical care setting. Infections by less common fungi may be particularly severe and rapidly progressive and require consultation with an infectious diseases specialist for appropriate treatment. Table 52.2 summarizes the most often encountered fungal diseases in the critical care setting with their generally accepted treatment options. Figure 52.1 presents our approach for the critically ill patient with fungemia. Table 52.2 Although C. albicans remains the most common pathogen in oropharyngeal and cutaneous candidiasis, non-albicans species of Candida are increasingly frequent causes of invasive candidiasis.101 Guidelines and reviews for therapy of candidiasis in the intensive care setting have recently been published.102–105 These guidelines are extensive and will not be repeated in detail. Rather, the text will focus on several clinical situations in which candidal infections are particularly challenging for the critical care specialist. The diagnosis of disseminated candidiasis is always a challenge.106 There is no single tool that conclusively makes this diagnosis. Isolation of Candida from the bloodstream is simply the most obvious form of disseminated candidiasis, but clinical experience makes it obvious that disseminated candidiasis can occur in the absence of detectable fungemia. Candidemia may be treated initially with either fluconazole, voriconazole, amphotericin B (or its lipid preparations), or an echinocandin. In the critically ill and unstable patient, an echinocandin or amphotericin B lipid preparations is preferred because of the broader spectrum of activity and more rapid onset of action.104,107 Owing to their greater safety, echinocandins are increasingly viewed as the initial agents of choice, and in fact, both the anidulafungin study and recent patient level meta-analysis of the candidemia clinical trials suggest that initial therapy with echinocandins may be superior to initial therapy with azoles.98,108 The susceptibility of Candida to the currently available antifungal agents can generally be predicted if the species of the infecting isolate is known.10,12,101,109–117 Bloodstream isolates of C. albicans, C. tropicalis, and C. parapsilosis are generally susceptible to fluconazole and amphotericin B. Isolates of C. glabrata and C. krusei will often (if not always, as is the case with C. krusei) be resistant to fluconazole. Isolates of C. lusitaniae may be resistant to amphotericin B. Antifungal susceptibility testing is becoming increasingly important as a guide in the treatment of these infections.118 In particular, detection of fluconazole resistance is very valuable, as it provides support for continued use of echinocandins or polyenes. Although all Candida spp. are generally considered to be susceptible to echinocandins, C. parapsilosis has higher minimum inhibitory concentrations (MICs) than the other Candida spp. This, however, has not translated into reduced activity in clinical trials.85,119 Once the organism is speciated, or if the prevalence of more resistant species is low, therapy may be switched to fluconazole. Fluconazole has demonstrated to be as effective in clearing candidemia as amphotericin B in immunocompetent hosts. Duration of treatment is 14 days following the last positive culture,21 and if a central line is present, removal is highly recommended. If evidence of disseminated candidiasis is found, therapy should be prolonged to at least 4 weeks to ensure proper organ clearance. Although long an area of contention, current data strongly suggest that candidemia is often related to (if not primarily propagated by) a central venous catheter. Central venous catheters in particular have been found to be both a risk factor for developing candidemia120–123 and associated with persistent fungemia.121 Removal of the catheter has been associated with shorter duration of subsequent candidemia124 and improved patient outcome.125,126 Unique to the species of Candida, candidemia with C. parapsilosis is almost always due to a catheter.127,128 The situation may be different for neutropenic patients, particularly those who have permanent, lower-risk catheters such as Hickman catheters. Such catheters may of course become infected, but these patients may also have candidemia due to entry of the organisms from the gut into the bloodstream. This concept is supported by demonstrations that Candida can enter the bloodstream from the gut,129 by the relative lack of effect of catheter removal in a large cohort of cancer patients,128 and by the frequent demonstration of gut wall invasion in patients who die with disseminated candidiasis.130,131 Unfortunately, there is no convincing way to tell if a given catheter is involved. Differential quantitative blood cultures through the line and from a peripheral site have been suggested to be one approach to resolving this problem,132 but this technique remains controversial.133 On a practical basis, serious consideration to line removal should be given if fungemia persists for more than a few days. A recent patient level meta-analysis of the major candidemia clinical trials has confirmed a mortality rate reduction and microbiologic cure benefit for this approach.108 Although there are many risk factors for development of disseminated candidiasis, colonization at one or more nonsterile sites represents an unusually strong risk factor.134 As discussed earlier, local oral and mucosal candidiasis should thus be considered as a predictor of possible invasive disease in the critically ill or immunocompromised host.135,136 Esophageal candidiasis does require systemic treatment. Fluconazole is generally preferred here, although amphotericin B and the candins can also be used. Treatment of asymptomatic candiduria produces only temporary clearing of the urine and is probably not indicated.22,23 However, candiduria should probably be treated in symptomatic patients, immunocompromised patients, low-birth-weight infants, renal transplant patients, and patients who will undergo urologic manipulation or surgery.102 If treatment is indicated, systemic therapy with amphotericin or fluconazole is preferred, and amphotericin bladder washes should be reserved for patients with renal insufficiency and low renal clearance. Removal of the urinary catheter is by itself a useful intervention and should always be considered. There are many other possible forms of invasive candidiasis: meningitis, endocarditis, and osteomyelitis, just to name a few. This area has recently been reviewed,103 and for most forms of this disease there are very few specific studies on therapy. The largest body of data will always be anecdotal reports of use of amphotericin B, and for essentially every form there are at least a few reports of successful therapy with fluconazole. In general, amphotericin B is preferred when the infection is most acute, when data on the nature of the infection are still being generated in the laboratory, or when the patient has previously received azole therapy. Fluconazole provides a good way to step down to an oral agent to complete therapy of infections due to susceptible isolates. Data for the echinocandins are starting to accumulate for these chronic infections.137 Removal of foreign body and standard surgical drainage are often key as well. An excellent example of the need to remove foreign bodies is found in treatment of dialysis catheter-related peritoneal candidiasis in which catheter removal is important. Surgical drainage is of course important in candidal peritonitis related to gut injury and fecal spillage. Treatment of cryptococcosis has recently been reviewed.138,139 Cryptococcal meningitis in non–human immunodeficiency virus (HIV)-infected adults continues to be seen sporadically. The majority of the published experience as to treatment is with amphotericin B given for 4 to 6 weeks.140,141 Because this therapy is curative in approximately two thirds of patients, this approach is warranted. Expert consultation is also appropriate for this relatively uncommon infection.142,143 On the other hand, cryptococcal meningitis in the HIV-infected patient is a well-established and common problem. Meningeal cryptococcosis in this setting should be treated with a 2-week course of IV amphotericin B or its lipid preparations,139,144,145 followed by life-long suppression with fluconazole.146,147 Current trends favor also using 5-FC unless there is a contraindication.50 Although itraconazole does not penetrate the CSF, anecdotal evidence has shown that it may be useful in treating CNS disease, although it is apparently less potent as a long-term therapy.148 For all forms of cryptococcal meningitis, intracranial hypertension should be aggressively treated with repeated lumbar puctures or a CSF shunt.149,150 The addition of steroids and other immune modulating agents to antifungal therapy has shown promising results in animal models,151 and clinical trials are being conducted. The immune reconstitution syndrome plays a major role in cryptococcal-related morbidity in HIV patients and the current recommendation is to delay antiretroviral therapy for a few weeks after treatment for cryptococcosis is started.139 Treatment of aspergillosis has been extensively reviewed.152–154 Invasive aspergillosis should be considered in any severely immunocompromised patient with an unexplained pulmonary or sinonasal process. Biopsy is normally required for definitive diagnosis, although a new generation of galactomannan-based serodiagnostic tests may prove useful as adjuncts to diagnosis.155,156 Although amphotericin B has classically been the initial treatment of choice for invasive aspergillosis, the current treatment of choice is voriconazole65,74 or a lipid preparation of amphotericin B. Aggressive surgical management is necessary and curative in some cases.106,157 Itraconazole has historically been an option for the treatment of aspergillosis in patients who are intolerant or refractory to amphotericin B, but newer and more effective azoles are now available.158 Overall, the outlook for invasive aspergillosis is critically dependent on recovery of immune function. Without this, the prognosis is usually dismal. Of note, recent studies have documented an increase in incidence of invasive aspergillosis in nonhematology, nontransplant patients seen in the intensive care unit (ICU), such as chronic obstructive pulmonary disease and autoimmune diseases.159,160 This trend should be monitored, and the critical care specialist should be suspicious when cultures of Aspergillus from a bronchoalveolar lavage or diagnostic markers such as galactomannan are positive.161 The initial treatment of choice for severe acute histoplasmosis is an amphotericin B preparation.162–164 Noncritical cases may be treated with itraconazole 200 to 400 mg/day. Fluconazole is only moderately effective and should not be used as primary therapy.165 Successful treatment results in a decrease of serum and urine Histoplasma antigen.166 Duration of therapy is a function of disease form and underlying immune status. HIV-infected patients with disseminated disease should be treated as acute histoplasmosis and maintained on life-long suppression with itraconazole. Liposomal amphotericin B has shown excellent efficacy in this setting.167 Non-HIV-infected patients may require from 3 to 12 months of therapy, and expert consultation is generally advised. The treatment of choice for mucormycosis (infections typically caused by Mucor spp., Rhizopus spp., and Absidia spp.) is aggressive surgical débridement and prompt start of high-dose amphotericin B or a lipid preparation.168 Follow-up therapy with itraconazole may be warranted, and expert consultation is advised. Posaconazole has shown efficacy in this setting.78 More recently, and despite the lack of in vitro activity of echinocandins against these agents, investigators have shown in vitro synergy and excellent clinical outcomes with the combination of amphotericin B and caspofungin.169 Correction of metabolic abnormalities (acidosis, iron overload, and hyperglycemia) should also be pursued aggressively. Other fungal infections, such as blastomycosis, fusariosis, and infection by Trichosporon spp., Coccidioides immitis, Malassezia furfur, and Penicillium spp. may be occasionally encountered in the critical care setting, particularly when caring for immunocompromised hosts. Although amphotericin B is probably the best empirical drug for any characterized suspected fungal infection, it is not very effective against some of these pathogens. Guidelines for treating some of these infections have been published.1 Expert consultation should be promptly obtained. Fever in the ICU patient is a complex problem that requires prompt evaluation for many possible sources, including infection, atelectasis, pulmonary embolism, drug fever, thermoregulatory dysfunction, and many more.170 Infection by Candida should be suspected when the patient has risk factors such as immunocompromise, broad-spectrum antibiotic therapy, parenteral nutrition, steroids, surgery (especially if the gut wall is transected), urinary catheters, and burns.171,172 Unfortunately, making a diagnosis of invasive candidiasis is difficult. In the most straightforward scenario, the patient is febrile and has positive blood cultures for Candida. On other occasions, biopsy or aspiration is used to make a clear-cut diagnosis of a localized abscess due to Candida. These situations are, however, the exception. Far more common is the scenario of a persistently febrile ICU patient with a combination of the previously mentioned risk factors. In this setting, we place great importance on the presence of positive cultures from nonsterile sites such as wounds, sputum, or stool. The key concept is that presence of Candida at any of these sites significantly increases the likelihood of developing invasive disease.134,173
Antifungal and Antiviral Therapy
Systemic Antifungal Agents
Polyenes
Amphotericin B and Its Lipid Formulations
Flucytosine
The Azole Antifungal Agents
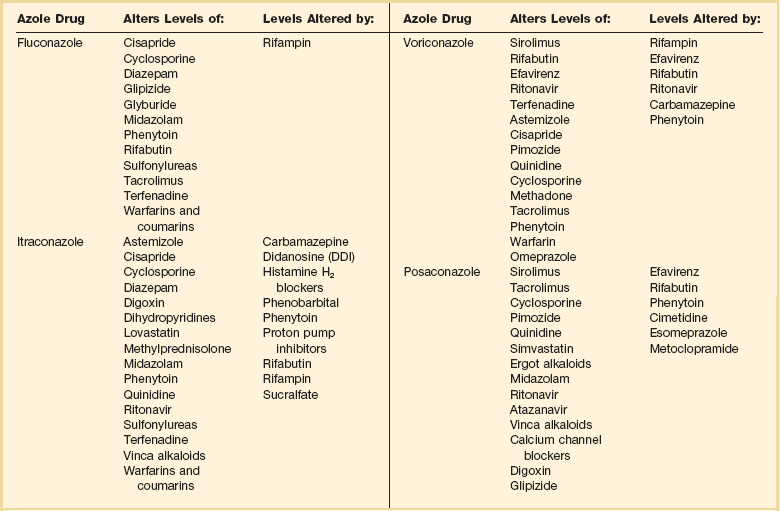
Fluconazole
Itraconazole
Voriconazole
Posaconazole
Echinocandins
Caspofungin
Micafungin
Anidulafungin
Specific Indications and Uses for Antifungal Therapy
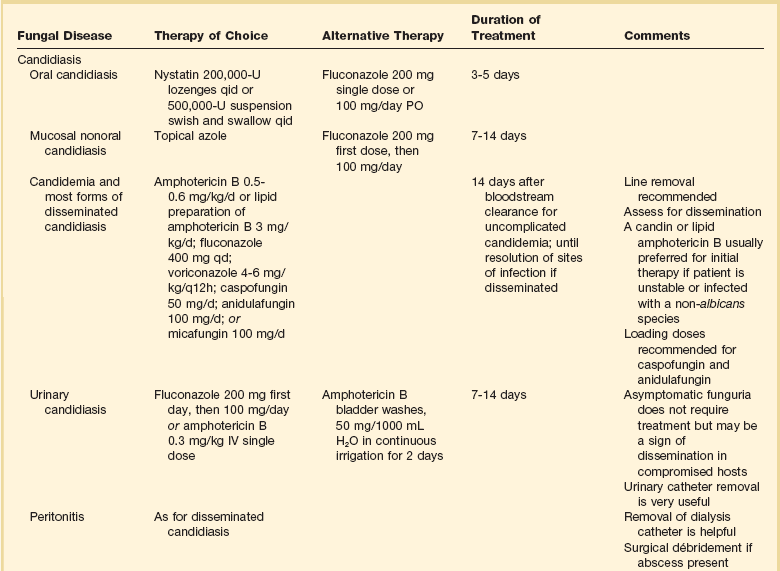
Candida Infections
Candidemia and Disseminated Candidiasis
The Intravenous Catheter in Candidemic Patients
Mucosal Infections and Colonization
Candiduria
Other Forms of Invasive Candidiasis
Cryptococcosis
Aspergillosis
Histoplasmosis
Mucormycosis
Other Fungal Infections
Areas of Controversy in Antifungal Therapy
Empirical Antifungal Therapy for the Febrile ICU Patient
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antifungal and Antiviral Therapy