172 Anticonvulsants
 Anticonvulsants: General ICU Concerns
Anticonvulsants: General ICU Concerns
Protein Binding
Drugs such as phenytoin, carbamazepine, and valproic acid are extensively protein bound, but only the unbound drug in the plasma is biologically active. Critically ill patients are often catabolic and have abnormally low circulating protein levels; thus, the concentration of unbound drug can be greater than anticipated despite a total serum (or plasma) drug level that is within the normal target range for the medication.1 Patients with hepatic and/or renal dysfunction are prone to discordance between total and unbound (free) serum levels. Routine monitoring of free drug levels is expensive but warranted in these patients. Unfortunately, most hospital laboratories routinely offer unbound serum levels for only one commonly used anticonvulsant, phenytoin.
Metabolic Derangements
Hyponatremia has been reported in patients who have been treated with carbamazepine, oxcarbazepine, and (rarely) other anticonvulsants. Anticonvulsant-induced hyponatremia has been attributed to the syndrome of inappropriate antidiuretic hormone (SIADH) (Table 172-1). Selected subgroups of patients are more at risk for anticonvulsant-induced hyponatremia, including elderly persons, menstruating women, patients who require administration of large fluid volumes, patients with renal failure, postoperative patients, and patients who are concurrently receiving other medications associated with hyponatremia.2
TABLE 172-1 Medications Associated with SIADH
| Barbiturates | Haloperidol |
| Carbamazepine | Chlorpropamide |
| Oxcarbazepine | Thioridazine |
| Thiazides | Imipramine |
| Vincristine | MAO inhibitors |
| Cyclophosphamide | Bromocriptine |
| General anesthetics | Oxytocin |
| Nicotine | Acetamides |
| Clofibrate | Tolbutamide |
| Nonsteroidal antiinflammatory drugs |
Adapted from Asconape J. Some common issues in the use of antiepileptic drugs. Semin Neurol 2002;22:27.
Drug Fever
Development of a fever coincident with initiation of an anticonvulsant in the ICU setting complicates patient management and is a serious potential concern. Drug fever is a particularly common occurrence with the two agents, phenytoin and fosphenytoin, but can occur with other anticonvulsants as well.1 Peripheral eosinophilia supports the diagnosis. However, it is frequently the case that the diagnosis of drug-induced fever is firmly established only when hyperthermia resolves after an alternative anticonvulsant is substituted for the original agent.
Alteration In Neurologic Examination
The toxic side effects of phenytoin or carbamazepine can promote development of ataxia. Valproic acid can induce tremors. Carbamazepine toxicity can present in a biphasic fashion (i.e., acutely and subacutely) as a consequence of increasing levels of a toxic metabolite.1
Renal Disease
Clearance of anticonvulsants can be significantly reduced when the glomerular filtration rate (GFR) falls below 10 mL/min. The clearance of phenobarbital and carbamazepine are not greatly affected by low GFR, but the clearance of phenytoin and valproic acid can be affected by changes in renal function. The higher protein binding exhibited by these latter agents makes measurement of the free levels of these drugs a better guide for dosage adjustments.1 Hemodialysis does not affect circulating phenytoin levels to a large extent, but renal replacement therapy can markedly affect serum levels of phenobarbital.
Drug Interactions
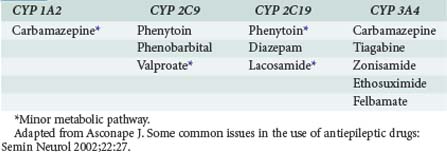
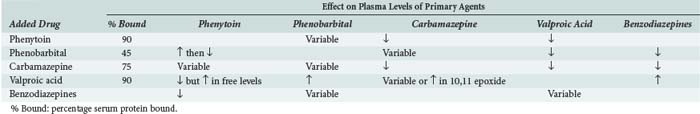
Many anticonvulsants can affect metabolism and or protein binding of other agents. Phenytoin, carbamazepine, and phenobarbital are all potent inducers of the hepatic P450 enzyme systems (Tables 172-2 and 172-3), and treatment with these anticonvulsants can affect the circulating concentrations of other medications (Tables 172-4 and 172-5) including concomitantly administered anticonvulsant drugs (see Table 172-5). Phenytoin can reduce the plasma concentrations of carbamazepine and valproic acid, whereas interaction with phenobarbital is variable. Phenytoin decreases the effectiveness of warfarin and theophylline. Valproic acid inhibits the metabolism of phenobarbital and carbamazepine (including its 10,11-epoxide metabolite), which can result in increased serum levels. Carbamazepine increases the hepatic metabolism of diazepam and valproic acid. Phenobarbital results in decreased circulating levels of warfarin, theophylline, and cimetidine.3 Cimetidine, amiodarone, isoniazid (INH), and chlorpromazine all decrease hepatic metabolism of many drugs including phenytoin (Table 172-6). Drugs that commonly decrease circulating phenytoin levels include digoxin, cyclosporine, corticosteroids, warfarin, and theophylline. Aluminum hydroxide, magnesium hydroxide, and calcium-containing antacids decrease the absorption of enterally administered phenytoin. Some of the newer anticonvulsants such as levetiracetam and lacosamide are excreted via the kidneys for the most part, and their circulating levels are unaffected by hepatic metabolism. In addition, drug-drug interactions are not a major concern with these newer agents, and they do not affect the levels of other anticonvulsants.
TABLE 172-3 Anticonvulsant Induction of Hepatic Metabolic Enzymes
| Inducers | Inhibitors | No or Minimal Effect |
|---|---|---|
| Carbamazepine | Valproate | Gabapentin |
| Phenytoin | Felbamate | Lamotrigine |
| Phenobarbital | Topiramate | |
| Primidone | Tiagabine | |
| Oxcarbazepine | ||
| Levetiracetam | ||
| Zonisamide |
Adapted from Asconape J. Some common issues in the use of antiepileptic drugs: Semin Neurol 2002;22:27.
TABLE 172-6 Common Drug Interactions of Anticonvulsants
| Phenytoin and Carbamazepine | ||
|---|---|---|
| Added Drug | Phenytoin | Carbamazepine |
| Salicylates | ↑ | |
| Erythromycin | ↑↑ | |
| Chloramphenicol | ↑ | |
| Trimethoprim | ↑ | |
| Isoniazid | ↑ | ↑ |
| Propoxyphene | ↑ | ↑ |
| Amiodarone | ↑ | |
| Diltiazem, verapamil | ↑ | |
| Cimetidine | ↑ | ↑ |
| Ethanol | ↓ | |
| Rifampin | ↓ | |
| Digitoxin | ↓ | |
| Cyclosporine | ↓ | |
| Warfarin | ↓ | |
| Theophylline | ↓ | |
| Glucocorticoids | ↓ | |
↓, decrease in plasma levels; ↑, increase in plasma levels.
Idiosyncratic Reactions
Hypersensitivity reactions are common with phenytoin and carbamazepine and can be manifested by fever, rash, and/or eosinophilia.1 Drugs associated with a high risk for the development of rash include phenytoin, phenobarbital, primidone, lamotrigine, carbamazepine, oxcarbazepine, and zonisamide4 (Table 172-7). Transient leukopenia and thrombocytopenia are commonly seen with carbamazepine and valproate. Other less common drug-related effects include hepatic failure, pancreatitis (valproic acid), agranulocytosis, aplastic anemia, megaloblastic anemia (phenytoin), Stevens-Johnson syndrome, and lupus-like syndromes. Although rare, severe hepatic dysfunction secondary to formation of a toxic metabolite can occur with valproic acid therapy. This potentially fatal reaction most often occurs in children younger than 2 years of age who are also receiving aspirin and other drugs for control of seizures.
TABLE 172-7 Antiepileptic Drugs and Risk of Skin Rash
| High Risk | Low Risk |
|---|---|
| Phenytoin | Valproate |
| Phenobarbital | Topiramate |
| Primidone | Gabapentin |
| Carbamazepine | Tiagabine |
| Oxcarbazepine | Levetiracetam |
| Lamotrigine | Lacosamide |
| Zonisamide |
Data from Asconape J. Some common issues in the use of antiepileptic drugs: Semin Neurol 2002;22:27.
Management Of Anticonvulsant Toxicity
Management of patients suffering from severe toxicity requires comprehensive supportive therapy including airway management, hemodynamic support, and oral administration of activated charcoal. Charcoal has been especially useful for managing cases of acute valproate acid intoxication.5 In cases of valproic acid or carbamazepine poisoning, concurrent hemoperfusion and hemodialysis to enhance elimination of the anticonvulsant can be useful when patients are hemodynamically unstable and the clinical condition is worsening despite aggressive supportive care.6
 Specific Anticonvulsant Properties by Class
Specific Anticonvulsant Properties by Class
Benzodiazepines
For immediate therapy, benzodiazepines are still considered first-line treatment for most seizures. These drugs are highly lipophilic, are potent γ-aminobutyric acid (GABA)-activated agonists, and serve to improve local inhibition of signal transmission. The most commonly used benzodiazepines in the ICU are diazepam, lorazepam, and midazolam. In the case of hepatic failure, oxazepam may be preferred because it is the only benzodiazepine not metabolized by the liver.7
Diazepam
Dosing
Pharmacokinetics
Midazolam
When a short-acting benzodiazepine is needed, most clinicians now employ midazolam instead of diazepam. Midazolam is highly lipophilic, and the onset of its effects occur very rapidly following IV administration.13 Midazolam is marketed as a water-soluble prodrug. Following IV administration, the drug is transformed into a lipophilic compound by virtue of rapid closure of the diazepine ring. Thus the drug is less irritating to veins than diazepam.
Dosing
Pharmacokinetics
Lorazepam
Lorazepam is the least lipid-soluble agent among the three commonly used benzodiazepines. As a consequence, the pharmacologic effects of lorazepam are delayed in onset and prolonged in duration.15 Lorazepam is ideally suited for acute therapy, together with longer prophylaxis against recurrence of seizures. In a 5-year randomized double-blind multicenter trial of four IV regimens for the treatment of generalized status epilepticus, Treiman et al. found that treatment with lorazepam (0.1 mg/kg) was successful in 64.9% of patients and significantly superior to phenytoin (P = 0.002) in a pairwise comparison.16 It is important to note that lorazepam’s longer duration of action can adversely impact the neurologic examination for several hours, potentially complicating medical management.
Dosing
Pharmacokinetics
Phenytoin
Phenytoin has been and remains the drug most commonly used in the ICU for prophylaxis against seizures. Several reasons for the continued popularity of phenytoin include its ease of administration, its availability in formulations suitable for either IV or enteral administration, its relative safety (severe toxic reactions are uncommon), and its efficacy against many seizure syndromes that occur in the ICU setting, including status epilepticus. Temkin et al. reported that prophylactic administration of phenytoin decreased the incidence of seizures during the first week following traumatic head injury by 73% compared to placebo.18 In light of its non-GABA-agonist action, phenytoin is not particularly effective against most drug-induced convulsions, especially those triggered by β-lactam antibiotics. Phenytoin is indicated for use against generalized tonic/clonic seizures and focal and complex-partial seizures. Phenytoin also is indicated for prevention of seizures following head trauma or elective neurosurgical procedures.
Dosing
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree