Fig. 52.1
This replica of Morton’s Inhaler shows the reservoir (globe) that contained the ether. The mouthpiece is to the left. A sponge was often placed in the globe, increasing the surface available for vaporization, but more importantly holding the ether so that liquid anesthetic would not be inhaled. (Courtesy of Wood Library-Museum of Anesthesiology, Park Ridge, IL.)
Morton’s carefully made device (he was late for the first demonstration because his instrument maker was adding last-minute improvements) provided no way of accurately controlling the ether concentration inhaled by the patient. It was made of glass, a poor conductor of heat. Cooling of the ether caused by its vaporization decreased the delivered concentration. Morton had little or no understanding of the physics involved. This limitation did not apply to the next character in our story.
News of the discovery of anesthesia quickly reached Britain. Physician John Snow appreciated the great significance of the discovery and set out to apply it in his practice. Unlike Morton, Snow understood the physical principles needed to deliver a controlled concentration of ether (or chloroform) and in 1847 constructed an elegant ether inhaler (Fig. 52.2). Air entered the vaporizer and passed through a metal spiral to maximize contact between the air and the ether, thus ensuring a saturated output (i.e., a constant output if the temperature was held constant). Snow immersed the vaporizing chamber in a water bath, to stabilize the temperature. He added a crude means (see below) to dilute the concentration delivered to the patient and connected the vaporizer to the patient with tubing of sufficient width to minimize resistance to breathing. This tubing was connected to a mask edged with leather that molded to fit the patient’s face. Some of these features in Snow’s vaporizer applied principles used in modern vaporizers.
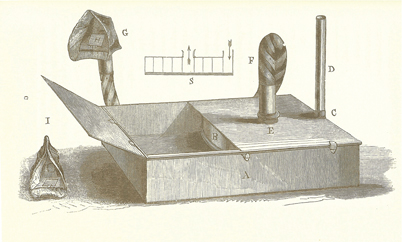
Fig. 52.2
Snow’s Inhaler. The air inlet was on the peripheral side (at the right). The drum contained a metal spiral with five turns. This allowed the air to become saturated with anesthetic vapor. Note the wide bore tube which extended from the center to the patient mask. (From Snow J: On the Inhalation of the Vapour of Ether in Surgical Operations: Containing a Description of the Various Stages of Etherization, and a Statement of the Result of Nearly Eighty Operations in Which Ether Has Been Employed In St. George’s and University College Hospitals. London: John Churchill; 1847. pp 1–88.)
Snow’s vaporizer allowed administration of a constant maximum concentration of anesthetic, useful for induction of anesthesia with ether, the agent for which it was originally devised. The high solubility of ether in blood limited the rate at which induction could be accomplished, and although high concentrations could irritate the airway, they hastened the process. Induction was the principal focus because early in the history of anesthesia, most operations were brief. The introduction of chloroform changed things. Airway irritation was minimal. Unlike ether, chloroform profoundly depressed the heart, making it important to not deliver a maximum concentration for too long. Snow solved this problem by adding a valve on his facemask that could be opened to dilute the delivered anesthetic with room air. Still, control over the delivered concentration was crude.
Joseph Clover solved some of these problems but added others. In 1877, he invented an “inhaler” for chloroform. By putting a measured amount of liquid chloroform into a large reservoir bag of known volume, he could produce a large amount of chloroform vapor in air at a known concentration. The bag was connected to a face mask, including a leaflet valve that allowed exhalation into the operating room. Air could be admitted from the room (thereby diluting the chloroform concentration) by turning the leaflet to the side. Interestingly, the mask and bag connectors had 22 mm diameters, the dimension used today and one that did not impose significant resistance to breathing. Unfortunately Clover’s device was cumbersome and had limited popularity. Clover died in 1882 and was buried 200 yards from Snow’s grave. One wonders what conversations they have when all is quiet.
Nitrous Oxide
Horace Wells had failed in his January 1845 attempt to demonstrate the anesthetic properties of nitrous oxide. The audience ridiculed Wells, calling the demonstration a humbug, and for nearly two decades nitrous oxide lapsed into obscurity. Gardner Colton resurrected nitrous oxide in the early 1860s. He gave it to more than 100,000 patients for dental procedures without, it is said, a fatality–a remarkable record.
Several problems surrounded the early use of nitrous oxide. First, in normal patients, the anesthetizing partial pressure exceeds atmospheric pressure. To achieve anesthesia, the earliest users administered 100% nitrous oxide, a lethal concentration if given for more than a minute or two because of the associated lack of oxygen. Nonetheless, nitrous oxide in air or occasionally 100% nitrous oxide for induction was used until the 1940s, with anesthesia resulting from a combination of nitrous oxide and hypoxia.
Second, because nitrous oxide was a gas, it could not be conveniently stored. It could be manufactured on the spot by heating (with great care, as the process could result in an explosion) ammonium nitrate in a retort [3]. The resulting nitrous oxide was purified by washing it in various reagents and stored in a reservoir’at first a bag made from oiled silk or animal bladders, and later in a gasometer (a small version of the enormous cylinders we see that are used to store natural gas). In 1865, the SS White Dental Manufacturing Co of Philadelphia made a storage bag. A valve was attached to the bag to control the release of the gas to the patient through a wooden mouthpiece. The patient held the mouthpiece with one hand while the nostrils were held closed with the other hand or a nose clip, in order to prevent air dilution. The clumsiness of this system limited its popularity.
The development of low pressure compressed gas cylinders in 1868 decreased the clumsiness; away with the cumbersome bladder/bag. In 1870, nitrous oxide was liquefied. Liquid nitrous oxide was supplied in cylinders by both Coxeter and Son, and Barth in Great Britain. By 1873, Johnson Brothers of New York were supplying similar cylinders to the American market. An attachment to the cylinder led to a large reservoir bag attached to a mask [4]. There was a supplemental bag, a valve to admit nitrous oxide directly into the mask, and an evacuation valve.
Why were these considerable efforts made, to overcome the difficulties and limitations imposed by the large volumes of nitrous oxide needed to provide anesthesia? Nitrous oxide offered two advantages over the then-popular ether: it acted quickly and didn’t irritate the airway. These properties complemented those of ether, reducing the slowness and untoward respiratory effects of ether, which were particularly problematic during induction. Around 1876, Clover designed a portable apparatus to deliver nitrous oxide and ether. A nitrous oxide cylinder supplied gas to an ether vessel (vaporizer) with a 6 liter bag connecting the vaporizer to a mask. Air or the nitrous oxide-ether combination could be admitted to the bag. Inadequate oxygen (hypoxia) evidenced by cyanosis caused the patient to breathe more, accelerating the uptake of anesthetic. When marked cyanosis occurred, fresh air was admitted by lifting the face mask.
A few perceptive people recognized the problems imposed by the lack of oxygen. It is not known when oxygen was first used with chloroform, but Snow used it in an unsuccessful resuscitation of a patient given chloroform. In 1868, Edmund Andrews, a Northwestern University surgeon, suggested adding oxygen to nitrous oxide. So did Paul Bert and Clover. In 1879, Bert combined 15% oxygen with 85% nitrous oxide to produce anesthesia in a pressure (hyperbaric) chamber. But such an approach was a logistical nightmare. A more practical solution was to go back to a combination of nitrous oxide, oxygen, and ether (called gas, oxygen and ether or GOE). Such a solution was not practical before 1885 when oxygen became available in cylinders. But GOE was not immediately adopted because the importance of using oxygen with nitrous oxide was not widely appreciated until after the turn of the twentieth century. Anesthesia providers continued to use nitrous oxide and air, not recognizing the potential negative effect on patient intelligence.
The availability of both nitrous oxide and oxygen in cylinders meant that large volumes of each could be stored efficiently, and this allowed development of apparatus delivering both. In 1886, Viennese dentist HT Hillischer produced the first machine dispensing both nitrous oxide and oxygen and coined the term “Schlafgas” (sleeping gas) to describe nitrous oxide. He found it best in most cases to commence with 10 percent oxygen/90 percent nitrous oxide, and to gradually increase this to 15 or even 20 percent oxygen. In dealing with alcoholic subjects and others resistant to the influence of nitrous oxide with 10 percent oxygen, he reduced the oxygen to 5 percent or even lower. If breathing became labored, or cyanosis appeared, he increased the percentage of oxygen. Hillischer used a proportioning valve to achieve the target percentages of oxygen. But his proportioning valve was a crude device. Something better was needed.
In 1885, SS White patented what today’s clinicians might recognize as an anesthesia machine. Gases were supplied from cylinders to separate inflatable bags then directed to a mixing chamber between the oxygen and nitrous oxide controls and delivered to the patient through wide bore tubing. The tap on the nitrous oxide bag needed to be fully opened, and a similar tap for oxygen allowed a variable flow according to uncalibrated gradations on a semi-circular gauge plate. By 1910 the SS White anesthesia machine had yokes for 4 cylinders, and reducing valves (pressure regulators) that decreased the high but variable pressure of gas from cylinders to a lower more constant level [5].
Gas Flow Measurement
Early anesthetic apparatus lacked a means to deliver precisely known flows (and therefore concentrations) of oxygen and nitrous oxide in the gases presented to the patient [6]. In 1902, dentist Charles Teter developed a gas machine that delivered a variable mixture of oxygen and nitrous oxide, each controlled by separate but coarse valves. There was no indicator of percentage or flow [7]. In 1916, after observing the principles of a mercury sphygmomanometer, Teter added mercury columns to his machine, calibrated to indicate gas flows.
Another dentist, Jay Heidbrink, purchased one of Teter’s machines in 1906, and set about improving it. He reasoned that better accuracy could be achieved by equalizing the pressure in the two bags supplying gas from the cylinders to the adjustable valves, and that the valves could be engineered to give finer control. An additional problem was moisture in the nitrous oxide, which often led to freezing of the nitrous oxide valve. Heidbrink solved this by placing an electric light bulb in the mixing chamber. Still, the machine had no indicator of gas flow. He achieved an acceptable calibration of flow control by adopting commercially available pressure-reducing valves and further refining the control valves. His first commercial machine was the Model A, introduced in 1912. He sold them for $ 1 per pound weight, $ 32 each.
In 1911, Frederick Cotton and Walter Boothby developed the first anesthesia machine that provided a visible indication of the rate of flow of nitrous oxide and oxygen. Each gas was fed separately into a water-filled glass mixing chamber. The rate of bubbling in the “bubble bottle” allowed an estimation of the flow and proportion of the gases. After exiting the first mixing chamber, a portion of the gas mixture could be directed through another chamber containing ether before rejoining the main gas stream.
In the following year, James Gwathmey improved on Cotton and Boothby’s idea by placing “bubble tubes” for each gas within the water sight feed bottle. Each tube had five holes, allowing from one to five streams of bubbles to be seen, thus indicating the gas flow. This was the forerunner of the 1917 Boyle apparatus, developed by Henry Boyle after meeting Gwathmey and purchasing one of his machines in 1912. James Gwathmey was one of the first physicians to practice anesthesia full time. He was president of the New York Society of Anesthesiologists, the forerunner of the American Society of Anesthesiologists.
Richard von Foregger (1872–1960), was born in Vienna, studied chemistry and emigrated to the US in 1898 [8]. In 1905, he began development of an oxygen generator using “oxone” or fused sodium peroxide. In 1907, he met Gwathmey, by which time the oxygen generator was a commercial success. In 1909, they produced an ether-oxygen device using an oxygen generator. On several occasions, Foregger and Gwathmey took the generator to Madison Square and administered oxygen to runners in 10-mile relay races and 6-day bicycle races. In 1914, Foregger established the Foregger Company and began manufacturing Gwathmey’s anesthesia machines.
Foregger later developed the water depression flowmeter (A competing CIG flowmeter is shown in Fig. 52.3). Gas flowing past a restriction in the top of the flowmeter depressed the water level in a tube submerged in a water-filled container, in proportion to the gas flow. This worked well as long as the flow did not exceed that which produced the maximum intended depression of the water level in the tube. Flows exceeding that limit forced gas into the water-filled container with a violence that depended on the flow. In some training programs no resident entered the “anesthetic brotherhood” until he had fully opened the oxygen valve to the flowmeter, blowing water all over the operating room.

Fig. 52.3
In these CIG water depression flowmeters, the water meniscus was at the black line just above the mark indicating a 1 liter per minute flow rate when there was no gas flow. Flow depressed the meniscus non-linearly, with the greatest change occurring at the higher flows (the opposite of what might be desired).
Dry Flowmeters
Karl Kuppers in Germany, introduced the rotating bobbin flowmeter, the “Rotamesser”, in 1909 for industrial purposes, and gynecologist Maximillian Neu used it in an anesthesia apparatus in 1910 [9]. It went into commercial production, but did not become widely known. Rotameters were not used again until 1937, when once more they were adapted to anesthesia machines, this time in Britain. American anesthesia providers working in British hospitals during World War II gained experience with rotameters and recognized their advantages. They were first used in American machines in 1950 by Foregger, and quickly became the gold standard for measuring gas flows.
In 1933, Heidbrink invented a tapered tube flowmeter (Fig. 52.4; really a variant of the rotameter), where a disc was attached to a stem, the disc rising in the tapered tube with increasing gas flows, the tip of the stem indicating the flow on a calibrated scale. It presented a graded visual display of flows for oxygen and nitrous oxide, the gases merging as they exited from the flowmeters. An improved version appeared on his Heidbrink machine in 1938.

Fig. 52.4
As shown on the oxygen flowmeter on the right, gas flow through these Heidbrink flowmeters caused the indicator to rise in the tube. Note that one flowmeter (flowmeter number 3 in the illustration) could be used for either helium or carbon dioxide
The Coxeter dry bobbin flowmeter (1933) consisted of a glass tube of uniform diameter with 24 small holes in the wall. An H-shaped bobbin in the tube rose with increasing gas flow, the flow exiting the tube through the holes. As flow increased, the bobbin rose and more gas left the tube through the holes. This flowmeter was relatively inaccurate because friction or dirt between the bobbin and the wall impeded the rise or fall of the bobbin. Since the bobbin moved in steps from hole to hole, it could not measure intermediate flows, and was inaccurate at low flows. These flowmeters were used in the 1933 Boyle machine that was the forerunner of the modern anesthesia machine. It is said that Boyle placed the oxygen flowmeter on the left because he was left handed. Machines manufactured in the US placed it on the right.
The Connell flowmeter was patented in 1934. The six-inch flow tube contained two ball bearings that rose with increasing flow inside a tilted glass tube with a tapered bore. Two ball bearings were needed to ensure steady movement and prevent each ball from oscillating in the tube. Gas flow was noted by the point where the two balls touched. Foregger also marketed a flowmeter that was inclined at an angle and used a ball indicator (Fig. 52.5). It also had shape-coded flow control knobs.
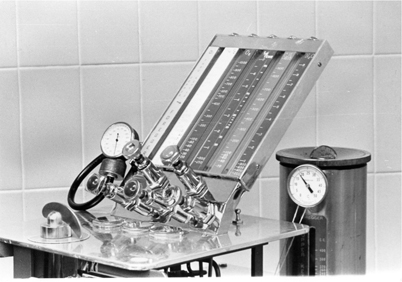
Fig. 52.5
In Foregger and Connell flowmeters one or two balls rose with increasing flow, and the flow was read at the juncture of the two balls. At the right is a Copper Kettle vaporizer. At the front of the machine is the on-off valve that controlled flow to the vaporizer. The flowmeter for the Copper Kettle is at the left. Note the thermometer at the top of the Copper Kettle
Ultimately, the rotameter became the most popular means of measuring gas flow in anesthesia, appearing on virtually all anesthesia machines up to the end of the twentieth century, when electronic measurement devices and displays replaced tapered tubes.
Intermittent Flow Machines: A Digression
The machines described above delivered a constant flow of gas, much of which was wasted [10,11]. Intermittent flow machines were devised to decrease this waste, and supply only what the patient needed. They delivered a controlled mixture of gases in a volume “demanded” by the spontaneously breathing patient. They featured a mixing device for oxygen and nitrous oxide. The McKesson Nargraf machine (see Fig. 6.3) was the most popular. It could be set to deliver an oxygen percentage from 0% to 100%. The negative pressure generated by the patient during inhalation initiated gas flow from the machine. The flow ended when the patient stopped inhaling. A one-way valve at the patient end of the breathing system opened, so that exhalation to the room could occur.
One of McKesson’s techniques, referred to as secondary saturation, used 100% nitrous oxide until severe cyanosis and muscular rigidity or spasm appeared. Oxygen was then added to the inhaled mixture. It was an exciting approach to anesthesia, but most anesthesia providers preferred not to bring their patients so close to death. Also, it was difficult to deliver a potent anesthetic [12]. Of note, with this apparatus and technique, anesthesia with halothane could be induced in 11 seconds. There is much more to the McKesson history, including the fact that the McKesson Nargraf machine in 1930 incorporated automated anesthesia record keeping.
The Evolving Anesthesia Machine
Anesthesia machines began to take the form initiated by the Boyle machine. In early machines, the Boyle bottle and direct-reading vaporizers like the Fluotec could be placed in the tubing between the machine outlet and the breathing system. If the gas flow in the fresh delivery tubing from the machine was attached (incorrectly) to the outflow connection to the vaporizer, a higher-than-expected output could result. In addition, these vaporizers handled the high flow from the oxygen flush poorly. Coxeters, the manufacturers of the Boyle machine, continuously modified and improved it. By 1927, the rubber hoses had been replaced by a large bore rigid tube between the flowmeters and the vaporizers. Later, the whole apparatus was incorporated into a table on wheels. The Boyle configuration persisted until the 1990s when major changes in anesthesia machines began to appear.
In 1978, Jeff Cooper and his colleagues at the Massachusetts General Hospital exhibited a completely computer-controlled anesthesia machine (the Boston Anesthesia System or MGH machine) [13]. It was never used for humans but was the first to suggest that a computerized machine was possible. In the 1990s anesthesia machines began to incorporate microprocessor-based technology. Computerized ventilators allowing the choice of various ventilatory modes appeared on many machines, and computer-controlled flowmeters working from flow sensors began to replace the rotameter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







