CHAPTER 103 ANESTHESIA IN THE SURGICAL INTENSIVE CARE UNIT—BEYOND THE AIRWAY: NEUROMUSCULAR PARALYSIS AND PAIN MANAGEMENT
MUSCLE RELAXANTS
Historic Perspective
One of the first documented uses of a neuromuscular blocking agent (NMBA) to aid in surgical closure of the abdomen occurred in 1912 in Germany.1 In the 1940s, the use of NMBAs during surgery started to become commonplace. Since the 1940s, the safety profile of NMBAs has significantly improved, and their use in the operating room (OR) has become routine. The introduction of mechanical ventilation in the intensive care unit (ICU) was closely followed by the use of NMBAs in the ICU. Most of our clinical experience with the use of NMBAs comes from the care of relatively healthy patients for a finite period of time in the OR. In contrast to patients in the OR, patients in the ICU are typically critically ill with multiple organ systems failing, require prolonged neuromuscular paralysis, and are receiving a large number of concomitant medications. Clinical studies have only recently started to examine indications for and complications of NMBAs in the ICU.
Current Epidemiology
Retrospective surveys reported by intensivists in 1991 and 1992 revealed that 10 patients per ICU per month required prolonged neuromuscular blockade.2 However, a study by Murray et al.3 in 1993 showed the probability of receiving a NMBA ranged from 0% in the neurosurgical ICU to 14% in the neonatal ICU. Several different situations have been identified that require short- or long-term neuromuscular paralysis.
Indications
Despite adequate sedation, some patients will not be able to tolerate mechanical ventilation, which can lead to unsafe peak inspiratory airway pressures. In a retrospective survey, Klessing4 reported that 89% of the cases requiring the use of NMBAs were related to the facilitation of mechanical ventilation, making it the most common reason for the use of neuromuscular paralysis. Other indications for NMBA use are the facilitation of endotracheal intubation, control of increased intracranial pressure (ICP), decrease of high muscle tone in certain medical conditions, and facilitation of needed medical procedures or diagnostic studies5 (Table 1). Short-acting NMBAs such as succinylcholine can be used to quickly secure an unprotected airway. However, many critically ill patients can have an airway secured by endotracheal intubation without the use of NMBAs. In patients with increased ICP, agitation or coughing caused by tracheobronchial suctioning can cause dangerous increases in ICP. Werba et al.6 demonstrated that pretreatment with vecuronium attenuated transient increases in ICP during suctioning.
Table 1 Indications for Use of Neuromuscular Blocking Agents in Intensive Care Unit
Concerns Regarding Overuse of Paralysis
Nevertheless, a retrospective study of 514 patients with severe head injuries demonstrated that the use of NMBAs resulted in longer ICU stays and increased morbidities.7 Symptomatic muscle rigidity found in tetanus, neuroleptic malignant syndrome, and status epilepticus can be ameliorated with NMBAs; however, treating the underlying cause of the symptoms is paramount. In addition, NMBAs can be used to facilitate minor bedside surgical procedures or diagnostic studies when immobility is critical for success, in particular in situations where adequate sedation levels to prevent movement cannot be achieved with analgesics and anesthetics alone, perhaps due to hemodynamic instability.
The long-term use of NMBAs has continued to increase over the last several years.8 New agents with improved safety profiles have been developed. A better understanding of the differences between surgical patients who are generally in good health and the critically ill patients found in the ICU is currently under active investigation. Appropriate medication selection will require a comprehensive understanding of the pharmacodynamics and pharmacokinetics of the various NMBAs as well as the physiologic status of the patient. Adequate sedation and analgesia are paramount because these patients are unable to communicate their distress. Sedation and analgesia and their monitoring are discussed further.
Mode of Action
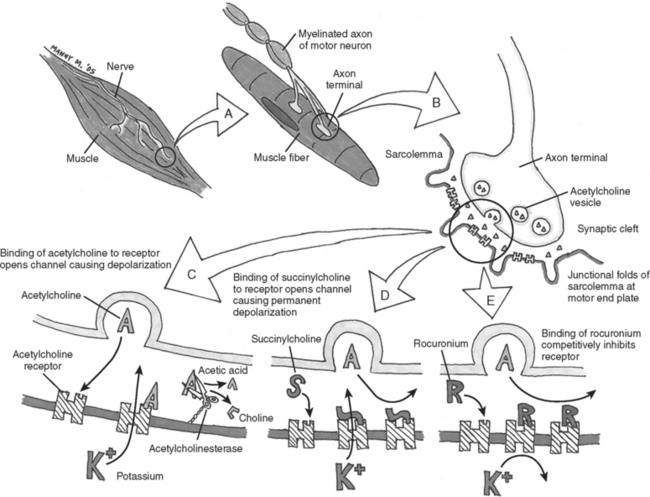
In general, NMBAs function by competing with acetylcholine (ACh) at the nicotinic cholinergic receptor (nAChR) at the neuromuscular motor end plate (Figure 1). NMBAs are classified as either depolarizing or nondepolarizing agents. The nondepolarizing agents are further divided into short, intermediate, and long acting, and classified according to their chemical structure as aminosteroidal or benzyl-isoquinoline compounds.9 The ideal muscle relaxant would have rapid onset, with predictable and controllable duration of action, and lack adverse effects on hemodynamics or significant toxicity. Its elimination should be independent of liver and renal function without accumulation over time. The ideal paralytic agent would have no interactions with other medications, as well as being cost effective with a long shelf life.
Depolarizing Neuromuscular Blocking Agents
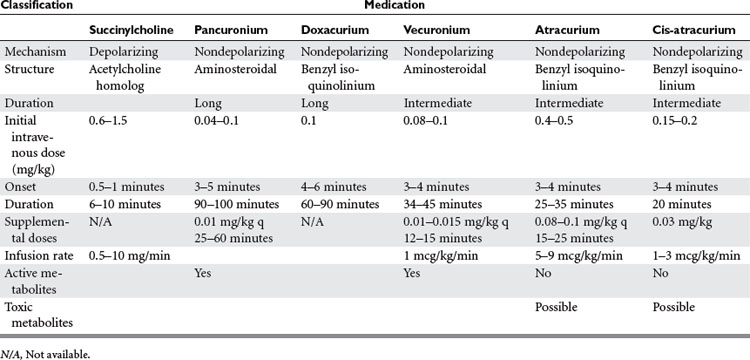
The only commercially available depolarizing NMBA is succinylcholine. Succinylcholine results in a lasting depolarization of the motor end plate, leading to an initial uncoordinated contraction of the muscle fibers, observed as “fasciculations,” but preventing subsequent activation. Through the continued stimulation of the nAChRs, repolarization of the receptors and subsequent muscle contractions are inhibited.9 The rapid onset and short duration of succinylcholine make it ideal for rapid-sequence intubations (dose 1–1.5 mg/kg IV bolus)9 (Table 2). But the concomitant potassium release as a result of the initial depolarization can be dangerous even in normal patients with borderline elevated potassium levels, but in particular in stroke, paralysis, burn, and spinal trauma patients 24 hours after the initial insult. This is because extrajunctional ACh receptors will have developed at denervated muscle fibers; they respond to succinylcholine by opening their potassium channels. Adverse effects of succinylcholine include the previously mentioned hyperkalemia, cardiac arrhythmias (in particular, bradycardia in children), myalgias, myoglobinemia, increased ICP, intraocular and gastric pressure, allergic reaction, and prolonged paralysis in patients with acquired or genetic plasma-cholinesterase deficiencies. Succinylcholine is a triggering agent for malignant hyperthermia in susceptible patients.
Nondepolarizing Neuromuscular Blocking Agents
Unlike succinylcholine, nondepolarizing NMBAs prevent muscle contraction by competitively inhibiting the binding of ACh to nAChRs. Structurally, the nondepolarizing NMBAs are classified as aminosteroidal compounds (vecurnonium and pancuronium) or as benzyl-isoquinolinium compounds (atracurium and cis-atracurium). Because of similarities in their chemical structure, various commonly used medications can imitate or alter the action of NMBAs or interfere with their elimination (see Table 2). Clinically the nondepolarizing NMBAs are classified by duration of action. ACh receptors are present in both the central nervous system (CNS) and the peripheral nervous system. Because nondepolarizing NMBAs are large charged molecules, they are unable to cross the brain–blood barrier into the CNS or enter across the placenta into the fetal circulation. Hence, ACh binds to receptors in the nerve ganglia and at the neuromuscular junction. NMBAs bind preferentially to the ACh receptor in the neuromuscular junction, but minimal interactions with the ACh receptors found at the autonomic ganglia help explain some side effects like the mild tachycardia observed with vecuronium and pancuronium or the bradycardia associated with the use of succinylcholine.
Pancuronium
Pancuronium (Pavulon®) is the principal long-acting aminosteroidal nondepolarizing NMBA used in the ICU. The chief advantages of pancuronium are its relatively low cost per dose and its comparatively long duration of action allowing for intermittent bolus dosing.8 Pancuronium is partially metabolized in the liver and excreted as the parent compound or as a metabolite by the kidneys. Significant renal or hepatic dysfunction may lead to prolonged paralysis. Other complications of pancuronium result from its vagolytic effect, which can result in tachycardia and hypertension.9
Doxacurium
Doxacurium (Nuromax®) is a relatively new long-acting benzyl-isoquinoline compound that causes little to no histamine release or cardiovascular side effects. In addition, duration of effect does not seem to accumulate with repeated dosing. Elimination appears to be independent of renal function.10
Vecuronium
Vecuronium (Norcuron®) is an intermediate-acting aminosteroid NMBA that is structurally related to pancuronium. The loss of a methyl group considerably decreases its vagolytic effect. Vecuronium is metabolized by the liver with three known metabolites retaining paralytic activity. The parent compound and its metabolites are excreted by the kidneys. Vecuronium can be administered either as intermittent IV boluses or as a continuous infusion.9
Atracurium
Atracurium (Tacrium®) and its isomer cis-atracurium (Nimbex®) are benzyl-isoquinolinium NMBAs. The advantage of these compounds is their elimination via Hoffmann degradation independent of both hepatic and renal function. Essentially, the drug spontaneously disintegrates in the warm physiochemical environment of blood (hence, unlike other NMBAs, atracurium needs to be cooled for storage). A metabolite common to both compounds is laudanosine, which in vitro has been shown to cause seizures. However, there are currently no documented occurrences of a seizure in a human receiving either of these medications. Cisatracurium is the cis-isomer of atracurium9 and as a result lacks the histamine release, the major side effect of atracurium. Both compounds can be administered as intermittent boluses or as a continuous infusion.
Monitoring of Neuromuscular Blockade
The primary use of NMBAs in the ICU is to facilitate mechanical ventilation.4 The degree of neuromuscular blockade required to reach this clinical goal will vary from patient to patient. The first set of guidelines detailing the use of NMBAs in the ICU was published in 1995 and later reevaluated in 2002. The current guidelines state that any patient receiving a NMBA should be assessed both clinically and by train-of-four (TOF) monitoring, with the clinical goal of NMBA titration to one or two twitches.11 Daily clinical assessment involves the observation of skeletal muscle movement and respiratory effort by the patient. Peripheral nerve stimulation by four equal electrical charges delivered at 0.5-second intervals can be evaluated with visual, tactile, or mechanical means (Figure 2). The TOF count is the observed number of twitches out of four. A TOF count of one out of four signifies that 90%–95% of the nAChRs are occupied. A TOF count of three out of four signifies that 75%–80% of the nAChRs are occupied. At 50% occupancy of the nAChRs, the patient can be safely extubated.12 This translates clinically to a sustained head lift for 10 seconds or to a train of four of four plus sustained tetanus to a 5-second stimulation with 100 Hz. Complete resolution of neuromuscular blockade is important not only to ensure adequate respiratory muscle power to sustain the work of breathing without ventilator support, but even more for the patient to protect the airway by unimpaired activity the bulbar muscles. Ulnar nerve innervation of the adductor pollicis muscle of the thumb is the most frequently used site to monitor TOF. The electrical stimulus should flow through the nerve, reach the neuromuscular junction, and release ACh, leading to a muscle contraction. If the electrodes are too close to the muscle tested, the current may directly cause a false-positive contraction. Hence any nerve/muscle pair that is at a certain distance apart can be used, such as the facialis/orbicularis oculi or the peroneus nerve/tibialis anterior. The monitoring of the degree of neuromuscular blockade may allow for the lowest NMBA dose and may minimize the adverse effects of prolonged NMBA use.11
Complications of Prolonged Neuromuscular Blockade
Complications related to the prolonged use of NMBAs include prolonged recovery from NMBAs, critical illness myopathy, and critical illness polyneuropathy.11 Prolonged recovery from NMBAs is defined as the time to recovery requiring 50%–100% more than predicted by pharmacologic parameters, and is most likely due to the accumulation of NMBAs or their metabolites (American Society of Anesthesiologists [ASA] guidelines). As detailed previously, the steroid-based NMBAs undergo hepatic metabolism yielding active metabolites. Interaction of the NMBAs or their metabolites with other concurrent medications may also explain the prolonged blockade (see Table 1).
Critical Illness Myopathy
Critical illness myopathy (CIM), also referred to as acute myopathy of intensive care and acute quadriplegic myopathy, is believed to be an acute primary myopathy. This myopathy results in diffuse weakness or flaccid paralysis with distal and proximal muscle groups equally affected. The muscle weakness persists long after the discontinuation of the NMBA and the elimination of the NMBA and its metabolites. The respiratory muscles are commonly involved, which prevents weaning from the ventilator.13 The majority of patients recover within 4 months; however, permanent neurologic deficits have been reported.14 An association of concurrent administration of NMBAs and corticosteroids with CIM exists.15–18 In addition, the prolonged use of both NMBAs and corticosteroids beyond 1–2 days increases the risk of myopathy (ASA guidelines). The ASA guidelines on the use of NMBAs in the ICU recommend that for patients receiving both NMBAs and corticosteroids, every effort should be made to discontinue the NMBAs as quickly as possible. The use of drug holidays may decrease the incidence of CIM.11
Critical Illness Polyneuropathy
Critical illness polyneuropathy (CIP) occurs due to diffuse axonal polyneuropathy. The mechanism is thought to involve impaired peripheral nerve perfusion, which leads to microvascular ischemia of the nerve.11,19 The electroneurographic pattern demonstrates a decrease in the amplitude of the nerve action potential with preserved normal conduction velocities. This condition is contrasted with Guillain-Barre syndrome (GBS), demyelinating polyradiculoneuritis, in which the amplitude of the nerve action potential is preserved and the conduction velocity is decreased.20 The occurrence of both CIP and CIM in critically ill patients is high; however, the occurrence of CIP has not been associated with the use of NMBAs (Table 3).
Table 3 Risk Factors for Critical Illness Myopathy and Polyneuropathy
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|