Anesthesia for Vascular Surgery
Todd J. Smaka
Timothy E. Miller
Michael P. Hutchens
Katie Schenning
Lee A. Fleisher
Tong J. Gan
David A. Lubarsky
Key Points
Related Matter
Aortic Cross Clamp for Blood Volume Redistribution
Vascular Disease: Epidemiologic, Medical, and Surgical Aspects
This chapter begins with a discussion of the pathophysiology of atherosclerotic vascular disease (AVD) and the general medical problems common in patients with peripheral vascular disease, particularly coronary artery disease (CAD). Organ protection strategies are then presented, with an emphasis on the heart and kidneys, among other organs. The specific surgical goals, anatomy, and complications for carotid, thoracic aortic, visceral, abdominal aortic, and lower extremity revascularization are placed in the context of optimal anesthetic management, including recognition of the ever-increasing use of endovascular techniques.
Pathophysiology of Atherosclerosis
Atherosclerosis develops as a response to injury. The primary injurious agents include lipoproteins containing apolipoprotein B, the most important of which is LDL. These lipoproteins filter into arterial intima through the endothelium. The entrapped lipoproteins then become modified into proinflammatory substances. In the subendothelial space enriched with atherogenic lipoproteins, most macrophages transform into foam cells. Foam cells aggregate to form the atheromatous core and as this process progresses, the atheromatous centers of plaques become necrotic, consisting of lipids, cholesterol crystals, and cell debris. Monocyte-derived macrophages act as scavenging and antigen-presenting cells and also produce several types of chemical mediators (e.g., cytokines, chemokines, growth regulating molecules) that are involved in inflammation. Adhesion molecules expressed by inflamed endothelium recruit leukocytes, including monocytes, which then penetrate into the intima, predisposing the vessel wall to lipid accretion and vasculitis.
The National Veterans Affairs Surgical Risk Study found that low serum albumin values and high American Society of Anesthesiologists physical classification were among the best predictors of morbidity and mortality after vascular surgery (Table 39-1).3 Risk factors for atherosclerosis include abdominal obesity, atherogenic dyslipidemia, hypertension, insulin resistance, proinflammatory state, and prothrombotic state. Major risk factors also include cigarette smoking, elevated LDL cholesterol (LDL-C), low high-density lipoprotein, family history of premature coronary heart disease, and aging; emerging risk factors include elevated triglycerides and small LDL particles. The relative contribution of these risk factors varies (Fig. 39-1).5
Table 39-1. The Ten Most Important Preoperative Predictors of Postoperative 30-Day Mortality after Vascular Surgery in Veteran’s Affairs Medical Centersa | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
Natural History of Patients with Peripheral Vascular Disease
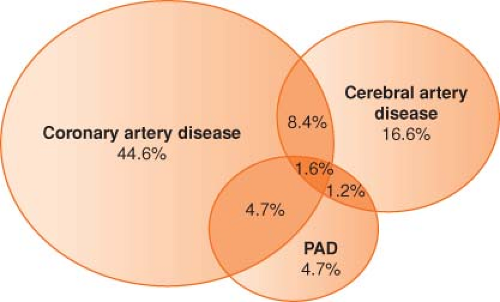
Atherosclerosis is one of the most important and common causes of death and disability in the United States and throughout the world. More than 25 million persons in the United States have at least one clinical manifestation of atherosclerosis. Throughout the past 50 years, coronary artery atherosclerosis has been a major focus for basic and clinical investigation. However, atherosclerosis must be recognized as a systemic disease with important sequelae in many other regional circulations5 (Fig. 39-2).
Stroke is the third leading cause of death and the principal cause of long-term disability in the United States. Six hundred thousand new or recurrent strokes occur annually. The risk of stroke is relatively uncommon (0.4% to 0.6% of patients) after noncarotid peripheral vascular surgery, but when it occurs it is associated with longer length of stay and higher mortality.6 The principal clinical syndromes associated with aortic atherosclerosis are abdominal aortic aneurysms (AAAs), aortic dissection, peripheral atheroembolism, penetrating aortic ulcer, and intramural hematoma. Patients with peripheral arterial disease (PAD), which is atherosclerosis affecting the limb, can develop disabling symptoms of claudication or critical limb ischemia. The prevalence of claudication is 2% among older adults, but 10 times as many elderly patients have asymptomatic lower extremity atherosclerosis. PAD affecting the lower extremities can be detected by the ankle–brachial index: The ratio of the highest systolic ankle blood pressure to the highest systolic arm blood pressure. The ankle–brachial index is the single best initial screening test to perform in a patient suspected of having PAD. The index is obtained with a blood pressure cuff and a hand-held continuous-wave Doppler. A ratio <0.9 is considered abnormal and <0.4 is often associated with limb-threatening ischemia.
Catheter-based angiography is the standard method for diagnosing the PAD, against which all other imaging modalities are compared for accuracy. However, recent advances in noninvasive angiography (magnetic resonance angiography and computed tomographic angiography) enable excellent noninvasive definition of the vascular anatomy. Carotid intima-media thickness is increasingly used as a surrogate marker for atherosclerosis. A meta-analysis found carotid intima-media thickness to be a strong predictor of future vascular events,7 though it does not significantly improve the Framingham and SCORE models for prediction of cardiovascular endpoints.8
AAAs occur in up to 5% of men older than 65 years; most of these aneurysms are small and require only infrequent follow-up. The risk of rupture is very low for AAAs ≤4 cm in diameter but rises exponentially for AAAs >5 cm. AAAs between 4 and 5 cm in diameter should be followed every 6 to 12 months to determine whether they are increasing in size. It is interesting to note that baseline hemoglobin concentration is independently associated with AAA size and reduced long-term survival following intervention for treatment. Thus the presence or absence of anemia offers a potential refinement of existing risk stratification methods.9
Medical Therapy for Atherosclerosis
Continuation of chronic medical therapy, including use of antihypertensives such as β-blockers and angiotensin-converting enzyme (ACE) inhibitors, statin drugs, aspirin, and control of hyperglycemia with hypoglycemics and/or insulin, may reduce perioperative morbidity and mortality in vascular surgery. Prevention of infection, including meticulous foot care in diabetic patients, is important to avoid tissue loss. Lifestyle changes such as weight loss and exercise can forestall claudication. The use of statin drugs may reduce progression or even cause regression of atherosclerotic plaques, improve endothelial function, and reduce cardiovascular events in high-risk patients. Patients with high cardiac risk undergoing vascular surgery who received preoperative statin therapy were less likely to die.10 Statin use is also associated with improved graft patency, limb salvage, and decreased amputation rate in patients undergoing infrainguinal bypass for AVD. Similar to β-blocker therapy, discontinuation of statin therapy for 4 days surrounding major vascular surgery is associated with an increased postoperative cardiac risk.11 ACE inhibitors have numerous beneficial effects in patients with AVD, including plaque stabilization. Cessation of smoking may be the most effective “medical” therapy.
Chronic therapy with aspirin or other anti-inflammatory drugs may retard the progression of atherosclerosis and prevent morbid cardiovascular events. A recent meta-analysis found the time interval between discontinuation of aspirin and occurrence of vascular events to be 14.3 ± 11.3 days for acute cerebral events, 8.5 ± 3.6 days for acute coronary events, and 25.8 ± 18.1 days for acute peripheral arterial syndromes.12 The recently reported RECO study suggested that discontinuation of aspirin for more than 5 days in patients with coronary stents is a major predictor of adverse cardiac events.13 In patients undergoing peripheral vascular surgery, continuation of clopidogrel within 48 hours was not associated with an increased incidence of major bleeding.14 The STRATAGEM trial randomized patients using antiplatelet agents for secondary prevention of CAD to either continuation of aspirin or placebo from 10 days before until the morning of surgery.15 Patients with coronary stents were excluded. There was no significant difference in the composite outcome of major thrombotic and bleeding adverse events occurring within 30 days of surgery. The use in cyclooxygenase 2 (COX-2) inhibitors of patients with AVD is unclear at present, with studies suggesting increased cardiovascular events with long-term use.16 In general, patients should continue to take aspirin until the day of surgery for carotid and lower extremity surgery, and individualize the choice for larger operations. In urgent situations when patients develop acute ischemia, systemic anticoagulation may be instituted.
Chronic Medical Problems and Management in Vascular Surgery Patients
Coronary Artery Disease in Patients with Peripheral Vascular Disease
Recently, two distinct types of PMI: “Early” and “delayed” occurring after vascular surgery have been identified. Early PMI resembles that of acute nonsurgical MI and is probably due to acute coronary occlusion resulting from plaque rupture and thrombosis. The “delayed PMI” is associated with sustained elevation of heart rate, absence of chest pain, and prolonged premonitory episodes of ST segment depression before overt MI. The delayed PMI resembles that resulting from increase in oxygen demand in the setting of fixed coronary stenosis.24 Myocardial oxygen supply may be diminished by anemia or hypotension, whereas oxygen demand may be increased by tachycardia and hypertension resulting from postoperative pain, withdrawal of anesthesia, or shifts in intravascular volume. Even small changes in cardiac troponin-I (cTnI) or cardiac troponin-T (cTnT) after surgery are associated with a worse perioperative and 6-month outcome, with a dose-response relationship.25 As a result, the new definition of MI requires the rise and fall of biochemical marker
of myocardial necrosis together with one of the following clinical or electrocardiogram (ECG) criteria: Ischemic symptoms, development of pathologic Q waves, ischemic ECG changes, or a coronary intervention.26 Troponin screening is now recognized as an effective means of surveillance for perioperative myocardial ischemic damage.27 In practical terms, the type of troponin that is used for surveillance, cTnI or cTnT is of little concern as both have similar diagnostic and risk stratification capabilities.
of myocardial necrosis together with one of the following clinical or electrocardiogram (ECG) criteria: Ischemic symptoms, development of pathologic Q waves, ischemic ECG changes, or a coronary intervention.26 Troponin screening is now recognized as an effective means of surveillance for perioperative myocardial ischemic damage.27 In practical terms, the type of troponin that is used for surveillance, cTnI or cTnT is of little concern as both have similar diagnostic and risk stratification capabilities.
Controversy persists as to whether preoperative cardiac risk identification benefits patients. However, in 2007, the American Heart Association and American College of Cardiology (AHA/ACC) revised their guidelines and classified the clinical predictors of increased perioperative cardiovascular risk (MI, CHF, and death) as “major,” “intermediate,” and “minor.”28 The major predictors also defined in the guidelines as “active cardiac conditions” are acute MI (<7 days), recent MI (7 to 30 days), unstable angina, decompensated CHF, severe valvular disease, and significant dysrhythmias. Active cardiac conditions, when present, mandate intensive management, which may result in delay or cancellation of surgery unless it is emergent. “Intermediate predictors” also defined in the guidelines as “clinical risk factors” are history of ischemic heart disease (e.g., current or prior angina pectoris or prior MI), past or compensated CHF, diabetes mellitus, renal insufficiency, or cerebrovascular disease. Minor predictors (recognized markers for cardiovascular disease that have not proven to increase perioperative risk independently) are age >70 years, abnormal ECG, rhythm other than sinus, and uncontrolled systemic hypertension. The guidelines place aortic and peripheral vascular surgery in the “high-risk” surgery category with an estimated cardiac risk (MI or cardiac-related death) exceeding 5%. CEA and most endovascular procedures are regarded as the “intermediate-risk” category, with an estimated cardiac risk ranging from 1% to 5%. With these definitions, the guidelines apply a stepwise approach to the evaluation of the patient incorporating clinical risk factors, exercise capacity (defined in terms of metabolic equivalents), and urgency of surgery (Fig. 39-5).
These guidelines have shifted the paradigm toward perioperative drug therapy aimed at reducing the cardiac risk either with no noninvasive cardiac testing or only very highly selective noninvasive testing when the outcome will result in a change in the anesthetic plan. Studies in which the AHA/ACC guidelines were used to guide preoperative testing have conflicted as to whether using the guidelines can improve outcome.
Preoperative Coronary Revascularization
Myocardial revascularization may have long-term benefits in patients with triple-vessel coronary disease or poor left ventricular function. However, mortality rates associated with these techniques are consistently higher in patients with peripheral vascular disease compared with those without. Whether preoperative coronary revascularization actually protects against perioperative cardiac events is controversial. The multicenter coronary artery revascularization prophylaxis (CARP) trial randomized patients with coronary disease (except left main disease or ejection fraction <20%) before elective vascular surgery to either coronary revascularization or medical therapy. With state-of-the-art aggressive medical therapy (>80% of patients on β-blockers, >70% on aspirin, and >50% on statins in both groups), they could find no benefit to coronary revascularization.29 A subsequent subgroup analysis of CARP trial examined the value of coronary artery bypass graft (CABG) versus percutaneous transluminal coronary angioplasty (PTCA) in those requiring coronary revascularization. Patients having a CABG had fewer MIs and tended to spend less time in the hospital after vascular surgery than patients having PTCA.30 In patients who underwent coronary angiography in both the randomized and nonrandomized portion of the CARP trial, only the subset of patients with unprotected left main disease showed a benefit with preoperative coronary artery revascularization.31 Thus, preoperative coronary revascularization (surgical or interventional) may be of no value in preventing cardiac events except in those patients in whom revascularization is independently indicated for acute coronary syndrome. High-risk patients should have surveillance for myocardial ischemia (typically troponin I or T) and risk-reducing strategies (including heart rate control). Should coronary revascularization be required before vascular surgery, then surgical revascularization is a suitable option compared with PCI. The safe time interval between surgical revascularization and vascular surgery is 4 to 6 weeks for surgical coronary revascularization and 2 weeks for coronary angioplasty. The safe interval for stents is much longer (see previous discussion).
The DECREASE trials evaluated the value of preoperative testing before major vascular surgery. At the time of the writing of this chapter, there is some concern regarding the validity of these trials, but no trial has been retracted. In DECREASE II, 770 patients having major vascular surgery and considered as having intermediate cardiac risk, defined as the presence of 1 or 2 cardiac risk factors, were randomized to either further risk stratification with stress imaging or proceed directly to surgery.32 All patients received preoperative bisoprolol with a targeted heart rate (HR) of 60 to 65 initiated before, and continued after surgery. The 30-day incidence of cardiac death and nonfatal MI was similar in both groups (1.8% in the no testing group vs. 2.3% in the tested group). The conclusion of the authors was that further risk stratification in this group of patients considered at intermediate risk based on clinical history alone was unnecessary as long as perioperative β-blockers were used, and testing only delayed necessary vascular surgery. In a pilot study (DECREASE V), 101 patients with three or more risk factors and a markedly positive stress test were randomized to coronary revascularization versus medical therapy. In those patients in whom there was successful revascularization, there was significant improvement in long-term outcome.33
Other Medical Problems in Vascular Surgery Patients
Correcting hypertension gradually over days to weeks before surgery allows for normalization of intravascular volume and cerebral autoregulation and results in a more stable perioperative course. However, a meta-analysis of 30 observational trials of cardiovascular outcomes after surgery in hypertensive patients suggests there is a statistically but “not clinically significant” increase in cardiovascular events for hypertensive patients.34
Undiagnosed diabetes and abnormal glucose tolerance are common in vascular patients, and predicts perioperative myocardial ischemia.35 Diabetic patients generally have a higher risk of MI and wound infection compared with nondiabetics undergoing AAA. Glucose management during carotid and thoracic aortic procedures may be especially important, in situations in which hyperglycemia may exacerbate neurologic injury. Intraoperative management of blood glucose concentration is an area of significant controversy, with some studies demonstrating benefit, particularly in cardiac surgery patients. However, two landmark trials of aggressive ICU glucose control have yielded opposite results, with the largest and most recent demonstrating increased mortality in patients whose capillary blood glucose concentration was kept below 110.36
Hypercoagulable states are more common in younger patients presenting for vascular surgery and in patients with vascular thrombi in unusual sites. Hypercoagulable responses to surgery may also predispose patients to vascular graft occlusion after surgery. Postoperative abnormalities include elevated fibrinogen levels, antithrombin III deficiency, impaired fibrinolysis, protein C deficiency, and protein S deficiency. Heparin-induced thrombocytopenia and thrombosis can occur (immunoglobulin G-mediated) after several days of exposure to heparin. Treatment includes cessation of all heparin, full anticoagulation with a direct thrombin inhibitor, and 3 weeks of warfarin therapy to prevent arterial thrombosis. Warfarin therapy alone is not recommended as it diminishes protein C and S activities and may initially promote thrombosis.
Organ Protection in Vascular Surgery Patients
Ischemia-reperfusion Injury in the Vascular Surgery Patient: Fundamental Concepts
Ischemia-reperfusion injury (IRI) is the sine qua non of organ failure in vascular surgery patients, and the techniques inherent to vascular surgery create a highly specific milieu that promotes multiorgan ischemia-reperfusion. It is important to recognize that IRI is an active, biphasic process (ischemia and reperfusion) in which both processes contribute to injury. Ischemia/hypoxia directly activates antiapoptotic, cell survival, and cell proliferation pathways in many cell types, including vascular endothelial cells and organ cell populations.37 These pathways are rapid, nontranscriptional, and transcriptional—thus they change the cell and organ functions within minutes, and the altered response continues for hours to days. In addition, hypoxia, particularly if prolonged, results in mitochondrial failure and cell necrosis which present neighboring cells with cytotoxic challenges. The placement of a vascular clamp directly injures perivascular endothelium and intima, including endothelial cells. Injury and activation of endothelial cells triggers both endothelial and nonendothelial responses, including immune/coagulation activation and other potent processes. Finally, reperfusion creates shear
stress as well as other changes that further activate endothelium and may induce other injurious or dysregulated processes. Overall, IRI leads to immune activation and translocation of immune/inflammatory cells such as macrophages, vascular paracrine dysregulation via secretion of vasoconstricting and vasodilating agents (endothelin-1, NOS, epoxyeicosatrienoic acids), and massive, dysregulated release of inflammatory cytokines.
stress as well as other changes that further activate endothelium and may induce other injurious or dysregulated processes. Overall, IRI leads to immune activation and translocation of immune/inflammatory cells such as macrophages, vascular paracrine dysregulation via secretion of vasoconstricting and vasodilating agents (endothelin-1, NOS, epoxyeicosatrienoic acids), and massive, dysregulated release of inflammatory cytokines.
Thus, IRI, particularly in vascular surgery patients, is a highly complex micro- and macrophysiologic process. This helps explain why single interventions aimed at single components of this process (e.g., increasing blood pressure to maximize flow) have proven ineffective in clinical studies. More importantly; however, this complexity is the reason why it is important to understand the rationales behind the many potential interventions.
Prevention of Myocardial Injury
Pharmacologic Approaches
Table 39-2. Pharmacologic Prophylaxis Against Acute Vascular Events in Patients Undergoing Vascular Surgery | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Commonly employed strategies for β-blocker administration in vascular surgery patients initiate oral therapy 7 to 30 days before with either atenolol 50 to 100 mg daily or metoprolol 25 to 50 mg (either extended-release once daily, or immediate-release twice daily). The intraoperative and postoperative periods are managed by intravenous administration of metoprolol titrated to heart rate and blood pressure. Alternatively, esmolol 50 to 500 μg/kg may be given intravenously over 1 minute followed by infusion of 50 to 300 μg/kg/min to achieve the target heart rate. It is important to recognize that some preparations of intravenous esmolol are dilute enough that administration of high dose infusions over 10 or more hours can result in hypervolemia and CHF, a complication that has been observed by the authors. In patients already taking β-blockers, they are continued to the day of surgery and followed by intravenous therapy to achieve the target heart rate as described previously.
α2-adrenergic agonists (clonidine or mivazerol) have been investigated as cardioprotective agents in the perioperative environment. The data supporting these medications are intriguing but not yet supported by large randomized trials. A meta-analysis suggests beneficial effects of perioperative α2-agonists with regard to cardiac morbidity and death in patients undergoing vascular surgery.41 This analysis included dexmedetomidine (DEX), which was not beneficial in its own meta-analysis,42 perhaps suggesting the effects of other α2-agonists might be beneficial if assessed alone. The authors have evaluated transdermal clonidine (0.2 mg/day) initiated the night before surgery, and 0.3 mg oral clonidine administered 60 to 90 minutes before surgery. Clonidine reduced intraoperative myocardial ischemia and catecholamine levels as measured on the first postoperative day.43
ACCF/AHA guidelines and an increasing volume of data support consideration of HMG-CoA reductase inhibitors (statins) for primary prevention of perioperative MI in vascular surgery patients. Two randomized trials have demonstrated reductions in adverse cardiac outcomes with statin use (atorvastatin 20 mg daily started 30 days before surgery or fluvastatin 80 mg daily started 37 days before surgery, both continued for 2 to 4 weeks after surgery).44,45 In addition, a meta-analysis of multiple observational trials found that preoperative statin therapy was associated with 59% reduction in relative risk of mortality after vascular surgery while no definitive conclusions could be drawn on cardiovascular morbidity.10 Preoperative extended-release fluvastatin may be of specific benefit to patients undergoing intra-abdominal vascular surgery who often have postoperative ileus. It is unclear why statins appear to be beneficial, but pleiotropic (nonlipid lowering) effects are thought to be involved. Finally, discontinuation of statin therapy (>4 days) after major vascular surgery is associated with an increased postoperative cardiac risk, suggesting that statin therapy should be resumed early after major vascular surgery.11 The recent ACCF/AHA guidelines advocate continuing statin therapy in patients currently taking statins as a Class I indication.22
ACE inhibitors also have beneficial actions with regard to acute vascular events independent of their antihypertensive action in patients with AVD. A single retrospective analysis of 3,020 patients undergoing vascular surgery at Veteran’s Administration hospitals suggests that ACE inhibitor therapy, in combination with other cardiovascular agents, may reduce perioperative mortality after vascular surgery.46 However, their use in vascular surgery is complicated by their potent perioperative hypotensive effects, and one trial of in excess of 800 patients found worsened outcomes in AAA surgical patients who received preoperative ACE inhibitors or angiotensin II receptor blockers (ARBs).47
A meta-analysis suggested beneficial effects of calcium channel blockers in reducing perioperative adverse cardiac events (cardiac-related death, MI, ischemia, or supraventricular tachycardia) in patients undergoing noncardiac surgery, with limited numbers of vascular surgery patients.48 The majority of these effects were attributable to diltiazem (one of two nondihydropyridine agents). Conversely, the dihydropyridine–calcium channel blockers have been associated with increased perioperative mortality.49 It seems clear that further evaluation of diltiazem as a cardioprotective agent is warranted, but to date no randomized trial has been conducted. On the basis of the evidence, the authors recommend that calcium channel blockers not be employed for perioperative cardioprotection.
Because it is a coronary artery dilator, nitroglycerin may seem attractive for perioperative prevention of coronary events. However, use of nitroglycerin may provoke or exacerbate hypotension with reflex tachycardia, or simply hypotension in the β-blocked patient. Several small controlled studies have shown minimal or no effect.50,51,52 Therefore prophylactic nitroglycerin should not be contemplated as a cardioprotective strategy.
Choice of anesthetic technique is widely believed to influence cardiac outcomes, although data specific to vascular surgery patients is scant. High-dose narcotic anesthetics reduce the stress response after major surgery, but mandate overnight ventilation, and increase costs and the risk of ventilator-associated adverse outcomes. Volatile anesthetics promote preconditioning, reduce troponin release, hasten extubation and hospital discharge in cardiac surgery, as well as reducing death and MI compared with intravenous anesthetics.53,54 Whether these findings are transferable to vascular surgery is unclear; however, and the best available data, a single randomized controlled trial of 88 patients, demonstrated no effect on troponin or mortality.55
Nonpharmacologic Approaches
Epidural analgesia may reduce perioperative myocardial ischemia because preload and afterload are reduced, the postoperative adrenergic and coagulation responses are reduced, and with thoracic administration, the coronary arteries are dilated. Although effects on cardiac outcomes in small trials have been mixed, a meta-analysis of these trials suggests that thoracic epidurals may reduce postoperative MI in high-risk patients undergoing major abdominal and vascular surgery.56 In addition, a Cochrane review of epidural analgesia for abdominal aortic repair found a statistically significant reduction (with relative risk 0.5) in postoperative MI in the treatment group, an effect which was more pronounced in the thoracic epidural (vs. lumbar) group.57 No randomized trial data exists to support the choice of epidural analgesia for open abdominal surgery; however, available data suggest it may be beneficial.
Anemia is an independent predictor of adverse short- and long-term cardiac outcomes in vascular surgery patients.58,59 However, it is important to recognize that red blood cell transfusion represents far more than simple treatment of anemia—rather it is administration of a highly active biologic agent with pleiotropic effects. In general, vascular surgery patients are at high risk for myocardial ischemia and this has resulted in a more liberal transfusion strategy in this population at most centers. However, as in other surgical populations, and even after adjusting for propensity and severity of illness, transfusion worsens important outcomes including survival after vascular surgery.60 In addition, emerging data also undermines the notion that transfusion improves outcomes in patients with myocardial ischemia and MI61 and may support the idea that restrictive transfusion practices are safe for critically ill patients with known CAD. In contrast, Beattie et al.62 used a propensity matched design of patients undergoing surgery from his center and found that β-blocked patients do not tolerate surgical anemia when compared with patients who are naive to β-blockers. Therefore, it may be critical to maintain adequate
hemoglobin levels in β-blocked patients. It is important to recognize that all packed red cell units are not the same, and that storage time may be directly related to adverse outcomes.63
hemoglobin levels in β-blocked patients. It is important to recognize that all packed red cell units are not the same, and that storage time may be directly related to adverse outcomes.63
Postoperative management, beginning in postanesthesia care unit (PACU), may affect cardiac outcomes. Maintenance of normothermia is cardioprotective,64 and should be pursued unless whole-body hypothermia is being employed as an organ-protective strategy (e.g., after cardiac arrest). Whether hypothermia is intended or not, its adrenergic consequences (shivering, tachycardia, hypertension) should be treated. In addition, aggressive postoperative surveillance may reduce cardiac risk as suggested by Monte Carlo simulation studies.65
Finally, the emergence of EVAR has prompted the hope that this procedure might reduce cardiac risk relative to open repair. Unfortunately, trials of open versus endovascular repair (OVER) have generally found no significant difference in the rate of cardiac complications. However, one randomized trial of 881 patients found a trend toward more events in the open repair group,66 and a more recent study in patients of low to moderate risk found that EVAR was associated with a lower risk of minor, but not major cardiac complications.67 Given the size of these studies, it is unlikely that EVAR will prove a very significant cardiac risk reduction when compared with open repair.
Prevention of Kidney Injury
Etiology and Epidemiology of Kidney Injury
Perioperative acute kidney injury (AKI) is a common complication of vascular surgery and is associated with high morbidity and mortality. The incidence of AKI is between 16% and 22% of patients undergoing aortic surgery.68 Postoperative mortality is four- to fivefold higher in those who develop AKI when compared to those who do not and minor elevations in serum creatinine are related to increased morbidity and mortality.69
The pathophysiology of perioperative AKI is multifactorial, with renal IRI playing a major role. In addition to IRI, the use of nephrotoxic drugs such as ACE inhibitors, nonsteroidal anti-inflammatory drugs (NSAIDs), aminoglycosides, and diuretics in the perioperative period can contribute to AKI. Other processes contributing to perioperative AKI during vascular surgery include atheroembolization to renal arteries during surgical manipulation of the aorta and surgical injury to the renal arteries themselves. In addition, rhabdomyolysis secondary to injury or immobilization is common in vascular surgery patients.
Preoperative renal dysfunction is the most powerful predictor of postoperative renal dysfunction. Patients with pre-existing renal insufficiency have an increased risk of postoperative renal failure, as well as cardiac complications and death. If patients receive chronic dialysis treatments, they should receive dialysis on the day before or the same day as surgery. Some patients will actually be hypovolemic as a result, which can contribute to hypotension with induction of general or regional anesthesia. Interestingly, women have an increased incidence of perioperative AKI than men following cardiac and vascular surgeries. This is in contrast to the general surgical population including all age groups, in which women seem to be protected from AKI when compared to men.70 Of note, women undergoing cardiac and vascular surgeries are older and more likely to be postmenopausal when compared to other women having surgeries, suggesting that estrogen plays a role in this protection.71
Several intraoperative factors including hemodynamic instability, the need for inotropic support, and the transfusion of greater than five units of packed cells or autologous blood can predict postoperative kidney injury.72 In aortic surgery, the level of aortic clamping is correlated with postoperative kidney dysfunction, with suprarenal cross-clamping of the aorta placing the kidneys at the highest risk. With suprarenal occlusion, renal blood flow decreases by 80%. Even with infrarenal aortic clamping, renal blood flow is still reduced by 45%. These renal hemodynamic changes do not immediately revert after clamp release, and persist for at least 30 minutes beyond the systemic cardiovascular return to baseline. In addition, prolonged aortic cross-clamp time (ischemia time) is associated with elevated postoperative creatinine values.73 Interestingly, intraoperative urine output is not predictive of postoperative renal function.74
In thoracic aortic repair, there are studies that suggest that an endovascular rather than open surgery may decrease the risk of perioperative AKI, especially in those patients identified as high risk.
Pharmacologic Approaches
Mannitol is widely used in vascular surgery because it induces osmotic diuresis, decreases epithelial and endothelial cell swelling, acts as a hydroxyl free radical scavenger, and increases synthesis of prostaglandin resulting in renal vasodilation. However, there is no evidence of a renoprotective effect of mannitol in vascular surgery patients. Similarly, loop diuretics are used intraoperatively to maintain urine output, but have not been shown to improve renal outcome or patient survival. Dopamine infusion of 0.5 to 2 ug/kg body weight per minute increases renal plasma flow, sodium excretion, and glomerular filtration rate (GFR). Unfortunately, multiple randomized trials and meta-analyses have failed to show benefit with regard to the outcomes of death or prolonged dialysis.75 Fenoldopam remains an active target of investigation and small studies have suggested benefit at the cost of increased complications (largely hypotension). Only one study has evaluated the renal effects of fenoldopam in vascular surgery, it found no renal benefit.76
Because of their anti-inflammatory and antioxidant effects, statins are being examined for their protective effects against perioperative AKI. A retrospective, population-based cohort study suggests that in patients older than 64 years undergoing major elective surgery, statin use decreased the rate of AKI and reduced perioperative mortality.77 Another retrospective cohort study observed that statin use decreased the incidence of kidney dysfunction following EVAR with suprarenal endograft fixation.78 Atrial natriuretic peptide (ANP) is also a candidate renoprotective agent which causes natriuresis, diuresis, and vasorelaxation. In a recent prospective, randomized, placebo-controlled trial, the intraoperative and postoperative infusions of ANP resulted in lower blood urea nitrogen (BUN), creatinine, and plasma B-type natriuretic peptide (BNP).79 However, trials of ANP for renal protection have suggested a trend toward increased mortality with ANP treatment.80
Nonpharmacologic Approaches
Multiple nonpharmacologic strategies have been employed to prevent renal injury during aortic surgery. Selective blood perfusion of the renal arteries has been attempted to minimize the time of renal ischemia during cross-clamping. Another strategy uses cold renal artery perfusion to produce local hypothermia to decrease renal oxygen demands. A randomized trial showed that
direct intra-arterial infusion of 4° C crystalloid or blood into the kidneys decreased the incidence of postoperative renal impairment.81 Remote ischemic preconditioning is thought to prevent IRI in multiple organ systems by inducing ischemic-protection pathways. Ali et al.82 found that intermittent cross-clamping of the internal iliacs reduced the incidence of renal insufficiency by 23%. However, repeated clamping of nonoperative arteries in patients with severe atherosclerosis likely increases the risk of surgical adverse events, and significant benefit would need to be demonstrated before adoption of such a practice.
direct intra-arterial infusion of 4° C crystalloid or blood into the kidneys decreased the incidence of postoperative renal impairment.81 Remote ischemic preconditioning is thought to prevent IRI in multiple organ systems by inducing ischemic-protection pathways. Ali et al.82 found that intermittent cross-clamping of the internal iliacs reduced the incidence of renal insufficiency by 23%. However, repeated clamping of nonoperative arteries in patients with severe atherosclerosis likely increases the risk of surgical adverse events, and significant benefit would need to be demonstrated before adoption of such a practice.
It has been widely hoped that morbidity and mortality from EVAR would be reduced compared to open aortic aneurysm repair. The UK EVAR trials found no long-term difference in renal function between open repair and EVAR.83 EVAR decreases kidney IRI because of the elimination of aortic cross-clamping; however, contrast-induced nephropathy is a significant cause of kidney dysfunction. As in open aortic repair (OAR), preoperative kidney function is the major predictor of postoperative renal complications. Because the renal dysfunction in EVAR is believed to stem from the use of intravenous contrast pre- and intraoperatively, prevention strategies are aimed at minimizing contrast exposure. Magnetic resonance angiography or gadolinium-based contrast studies have been proposed for patients at risk for contrast-mediated nephropathy. The use of CO2 as contrast media and endovascular ultrasound should be used when possible to limit the use of radio-opaque dye. Allowing adequate time for renal recovery between preoperative studies and EVAR may be of benefit. Other strategies for renal protection from contrast include the use of N-acetylcysteine, sodium bicarbonate, and ensuring adequate hydration.84
Prevention of Pulmonary Complications
Pulmonary complications are common after major vascular surgery (10% to 30% of AAA patients) and are associated with increased mortality and length of stay.85 Perioperative interventions clearly influence postoperative pulmonary function and likely reduce the risk of postoperative respiratory failure, prolonged mechanical ventilation, and pneumonia in surgical patients overall. Although data specific to vascular surgery are lacking, many large studies of perioperative pulmonary complications include vascular surgery patients, in particular patients undergoing aortic repair.
The American College of Physicians guidelines for reduction of perioperative pulmonary complications identify abdominal surgery, surgery lasting more than 3 hours, emergency surgery, preoperative CHF, and preoperative chronic lung disease as significant risk factors for pulmonary complications. The most effective preventive measure is postoperative lung expansion, either continuous positive airway pressure (CPAP) or incentive spirometry. Anesthetic technique is as an independent risk factor for pulmonary complications, with general anesthesia conferring an odds ratio of 1.8.86 Although there is some heterogeneity in the overall data, the Cochrane Database analysis confirms that postoperative thoracic epidural analgesia reduces postoperative respiratory failure and duration of mechanical ventilation in patients undergoing abdominal aortic surgery.57
Since the initial 2,000 ARDSNET trial reported a mortality benefit of low tidal volumes (6 to 8 mL/kg)87 in patients with acute respiratory distress syndrome, there has been interest in extension of this benefit to the perioperative population. No studies address the clinical benefit of lung-protective ventilatory strategies in vascular surgery patients. However, indirect data are intriguing. Two small clinical studies of surgical patients (in one case prolonged procedures, and in the other esophagectomy with one-lung ventilation) found reductions in systemic or bronchoalveolar lavage inflammatory markers,88,89 and a study of cardiac surgery patients found that patients ventilated with low tidal volumes were more likely to be extubated by postoperative hour 6 and less likely to be reintubated.90 No significant data exists to suggest that such tidal volumes are harmful.
Choice of procedure and surgical technique may influence pulmonary outcome. Aortic clamp site influences the risk of postoperative pulmonary complications (suprarenal vs. infrarenal 25% vs. 12%).91 In randomized trials, EVAR has not demonstrated reduced risk of pulmonary complications when compared with OAR.
In short, postoperative lung expansion with CPAP or incentive spirometry and postoperative thoracic epidural analgesia reduce perioperative respiratory complications. There is some suggestion that lung protective ventilator strategies used intraoperatively may also be protective.
Protection of the Central Nervous System and Spinal Cord
Prevention of Perioperative Delirium
Delirium (sometimes called “central nervous system failure”) is a common central nervous system complication of vascular surgery. Aortic aneurysm surgery is an independent risk factor for delirium,92 which in the past has been under-appreciated as a serious complication leading to severe adverse outcomes. Delirium is an independent risk factor for short- and long-term mortality, increased ICU and hospital stay, and skilled nursing facility discharge.93 Unfortunately, there are no known effective perioperative interventions to reduce the incidence of delirium as a whole and few studies specifically address vascular surgery patients. However, the limited available data indicate that age, preoperative cognitive dysfunction, depression, previous amputation, alcohol abuse, and intraoperative blood transfusion are risk factors. Alcohol withdrawal remains a common cause of perioperative delirium. In patients who remain sedated postoperatively, the anesthesiologist’s detailed social history may provide the only available information about preoperative substance use.
Prevention of Perioperative Stroke
Stroke is nearly twice as common in patients who have undergone AAA repairs than in the general population as long as 20 years postoperatively.94 Open thoracoabdominal aortic aneurysm repair carries a higher risk of stroke than AAA repair, between 1.5% and 3.5%, whereas the overall risk of stroke from thoracic endovascular aortic repair (TEVAR) is 2.9%.95 Unfortunately, there is little supporting any preventive measure for stroke in vascular surgery patients (other than those undergoing CEA), and scant data for surgical patients overall. Patients with symptomatic carotid stenosis benefit from carotid revascularization before major vascular surgery. Other potentially modifiable risk factors include atrial fibrillation and discontinuation of antiplatelet therapy, which both increase the risk of perioperative stroke.96 However, the risks of active management of atrial fibrillation or continuation of antiplatelet therapy through surgery have not been specifically evaluated in vascular surgery patients.
Prevention of Perioperative Spinal Cord Ischemia
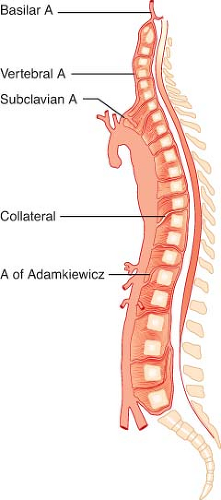
Spinal cord ischemia occurs in 1% to 11% of operations involving a distal aortic repair. The spinal cord is supplied by two posterior arteries; together, they supply 25% of spinal cord blood flow. The anterior spinal artery (Fig. 39-6) supplies 75% of spinal cord blood flow and is the primary supply to the anterolateral cord. The anterior spinal artery is fed by a series of radicular arteries arising from the aorta, and collateralization is poor. The blood supply to the thoracolumbar cord is derived from the radicular artery of Adamkiewicz. In 75% of cases, it joins the anterior spinal artery between T8 and T12, and in 10% it joins between L1 and L2. Much of the blood flow in the anterior spinal artery depends on the artery of Adamkiewicz. Because the flow in the spinal arteries depends on collateralization and is often bidirectional, the blood supply to the spinal cord can be shunted to the rest of the body when perfusion pressures are low. Such a situation may arise when a single high aortic occlusion clamp is applied.
Standard measures to prevent spinal cord ischemia include short cross-clamping time, maintenance of normal cardiac function, and high perfusion pressures. In high aortic clamping, other methods should be considered (Table 39-3). Some surgeons place a Gott shunt, a heparinized tube that can decompress the heart and also provide distal perfusion. The Gott shunt can be placed proximally into the ascending aorta (the most common site), aortic arch, descending aorta, or left ventricle, and inserted distally into the descending aorta (most commonly), femoral artery, or abdominal aorta. Even with a Gott shunt or partial bypass, there is an obligatory time of visceral ischemia when the visceral blood supply arises from a point between the proximal and distal clamps. Placement of a shunt may result in atheroembolism, which can produce rather than prevent ischemic injury and death. Other surgeons may place a temporary ex vivo right axillofemoral bypass graft before positioning for thoracotomy. After the thoracic aortic surgery is completed, the axillofemoral graft is removed. The placement of a shunt attenuates the hemodynamic response to aortic unclamping, reduces acidosis, and could conceivably ameliorate the hormonal and metabolic changes that accompany aortic occlusion.
Table 39-3. Methods of Spinal Cord Protection During Descending Thoracic Aortic Surgery | ||
|---|---|---|
|
Other groups have chosen to use partial bypass, either from the left atrium or ascending aorta to the iliac or femoral artery to provide distal perfusion and decompress the heart. A heat exchanger may be used to induce hypothermia, which may be neuroprotective. Segmental sequential surgical repair may minimize the duration of ischemia to any given vascular bed. Intercostal artery reattachment in hopes of preserving blood flow to the anterior spinal cord may be beneficial. After reperfusion, the heat exchanger can be used to warm the patient. Other potential advantages of left atrial–left femoral artery shunt with centrifugal pump support are
better operative field exposure, afterload reduction, maintenance of stable distal aortic perfusion, and reduced (but not eliminated) head and neck edema.
better operative field exposure, afterload reduction, maintenance of stable distal aortic perfusion, and reduced (but not eliminated) head and neck edema.
A markedly reduced incidence of neurologic deficits has been reported when distal aortic perfusion is combined with drainage of cerebrospinal fluid (CSF).97 CSF drainage is used in the hope of improving the pressure gradient, allowing spinal cord blood flow as aortic occlusion lowers distal arterial pressures and increases the central venous pressure. The new endovascular techniques represent an alternative therapy when anatomy permits; lower paraplegia rates have been reported compared with open surgery.98 However, neurologic complications still occur, and CSF drainage is commonly employed in both open and endovascular procedures for primary prevention and management.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree