Anesthesia for the Older Patient
G. Alec Rooke
Key Points
Related Matter
Age-Related Changes in Body Composition
Cardiovascular Response to Orthostasis
Age is not a particularly interesting subject. Anyone can get old. All you have to do is live long enough.
—Don Marquis
The above quote suggests that aging is dull. To many medical practitioners, it is far worse than “dull.” It is frustrating from its complexity of care, and discouraging in its monetary reimbursement. To those concerned with the federal budget, medical care for the aged threatens to bankrupt the nation. Nevertheless, the impact of aging on the practice of medicine is far-reaching and profound, and therefore cannot be ignored. Just as children are not “little adults,” the older patient is truly different from the younger adult counterpart. All caregivers, including anesthesiologists, should be knowledgeable of at least some aspects of aging in order to provide intelligent deviation from their standard practice. Basic information is more available than ever before, much of it electronically from the American Society of Anesthesiologists (www.asahq.org), the Society for the Advancement of Geriatric Anesthesia (www.sagahq.org), and the American Geriatrics Society (www.americangeriatrics.org). In reality, caring for an older patient is rarely dull, if for no other reason than their diverse and fascinating lives. Anyone with a passing interest in physiology
should enjoy the application of aging physiology to anesthetic management. Yes, their care is often time-consuming, stressful, and requires extra effort, but more often than not it provides the anesthesia caregiver the opportunity to truly practice medicine and make a positive impact on a vulnerable patient’s life.
should enjoy the application of aging physiology to anesthetic management. Yes, their care is often time-consuming, stressful, and requires extra effort, but more often than not it provides the anesthesia caregiver the opportunity to truly practice medicine and make a positive impact on a vulnerable patient’s life.
Demographics and Economics of Aging
I advise you to go on living solely to enrage those who are paying your annuities. It is the only pleasure I have left.
—Voltaire
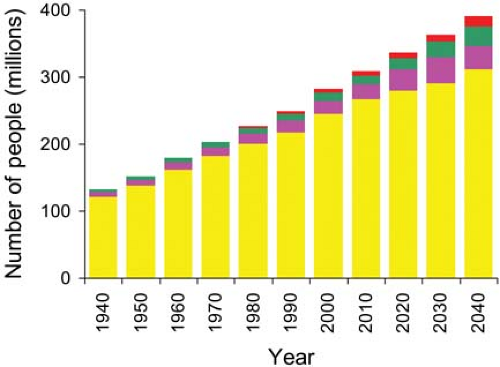
When Social Security was initiated in 1935, only 6.1% of the US population was older than 65 years.a By 2010 that percentage had grown to 13% and represented 40 million people. By 2030 it is expected to be nearly 20% of the US population and represent 71 million people. The percentage of the US population older than 85 is expected to double from 2010 (1.9%) to 2040 (estimated 3.7%). The growth of the US population and its older subgroups is shown in (Fig. 33-1). The impact of these statistics is enormous with respect to medical care. In 2007, patients over 65, 13% of the population, accounted for 43% of the total US 165 million inpatient days, a rate per capita 5 times greater than those under 65.1 Upon examination of the 2006 inpatient and ambulatory procedure data, there were found to be an estimated 73 million surgical and nonsurgical procedures performed in the United States after exclusion of procedures that were unlikely to have involved anesthesia services.2,3 Of these, 28% were on patients older than 65 years. Thus it appears that people over age 65 have surgery 2.7 times more often than people under age 65.
The Process of Aging
You can’t help getting older, but you don’t have to get old.
—George Burns
There are many theories of aging, and it is possible that each plays a role in the physiologic changes of aging and the inevitability of death. Some theories of programmed aging suggest that there are genetic codes that dictate how long a cell (and the organism as a whole) will live. This “killer gene” theory would have to confer an advantage to species survival for evolution to result in programmed death. Although this phenomenon does occur in nature (e.g., females of certain species of octopi), there is little evidence that such a mechanism applies to mammals. Another theory of limited cell viability is the attractive hypothesis of telomere shortening. Each time a cell divides, a telomere is cleaved off the DNA. When the DNA runs out of telomeres, the cell cannot divide and will eventually die.4 Such limited cell division occurs in cultured mammalian cells, and although species life span correlates roughly with the number of allowable cell divisions, there is little evidence that telomere shortening affects human life span. Furthermore, aging involves more than just the death of the organism.
Mammalian aging clearly involves a gradual, cumulative process of damage and deterioration. The question could be posed: Why is such a process allowed in nature? Teleologic reasoning would suggest that once offspring have been raised, there is no further need for continued survival of the individual. Protective mechanisms against aging are costly to the organism, so the “disposable soma” theory of aging states that antiaging mechanisms only need to be good enough to give the next generation the best opportunity to reproduce. In fact, most of the gains in average human life span have been as the result of reducing those factors that cause premature death: Predation, accidents, and disease. The inability to thwart aging completely implies that the average
human life span is limited, and that if everyone died only of “old age,” the age at death would end up being a bell-shaped curve centered at a certain value, probably around age 85.5 Nevertheless, it is possible that the bell-shaped curve could be shifting to a higher value, but how far it can be shifted is unclear.
human life span is limited, and that if everyone died only of “old age,” the age at death would end up being a bell-shaped curve centered at a certain value, probably around age 85.5 Nevertheless, it is possible that the bell-shaped curve could be shifting to a higher value, but how far it can be shifted is unclear.
Functional Reserve and the Concept of Frailty
Old age is no place for sissies.
—Bette Davis
Functional reserve represents the degree to which organ function can increase above the level necessary for basal activity. For healthy individuals, reserve peaks at approximately age 30, gradually declines over the next several decades, and then experiences more rapid decline beginning around the eighth decade. Assessment of reserve is something anesthesiologists perform all the time. For example, the ability to achieve a minimum of four metabolic equivalents appears to confer enough cardiovascular reserve to tolerate the stress of most surgical procedures.7 Even without formal assessment, an intuitive sense of reserve is often obtained through simple observation. A person who looks and acts old presumably has suffered more from the aging process, regardless of chronologic age. The loss of subcutaneous tissue, unsteady or slowed gait, decreased cognition or memory, a stooped body habitus, and minimal muscle mass all contribute to the impression of frailty. It turns out that some of these traits may well correlate with reserve. For example, sarcopenia is a serious problem for the very old, and when severe enough, can lead to an accelerated deterioration with further weight loss, mental and physical decline, and increased mortality.8 Indexes of frailty have been constructed and found to not only correlate with disability and mortality in community dwelling elderly, but also correlate with postoperative complications, especially the likelihood of discharge to a skilled or assisted living facility.9,10
Physiologic Age
If you didn’t know how old you were, how old would you be?
—James Hubert ‘Eubie’ Blake
The Physiology of Organ Aging
“If I’d known I was going to live this long, I’d have taken better care of myself.”
—James Hubert ‘Eubie’ Blake, age 100
Defining what constitutes “normal aging” is difficult. Is it what happens under the best of circumstances, or what happens to the “average” person? Comparisons of young and elderly subjects may not strictly reflect aging, as the elderly subjects may have experienced a much different diet, lifestyle, and environmental exposure than what the young group will experience by the time they become old. Following a group of healthy subjects over a long period is more likely to define the effects of aging, but not all available data come from such longitudinal studies. Studies that examine only the very old persons may actually underestimate the typical effects of aging because individuals generally do not achieve old age unless there is something intrinsically robust about them. Lastly, the reader is reminded that, as with the discussion of physiologic age, the effects of aging apply in varying degrees to any given patient, and that disease will interact with aging to further diminish functional organ reserve.
Changes in Body Composition, and Liver and Kidney Aging
I have everything I had twenty years ago, only it’s all a little bit lower.
—Gypsy Rose Lee
Liver mass decreases with age, and accounts for most, but not all, of the 20% to 40% decrease in liver blood flow.14 There is also a modest reduction in phase I drug metabolism and bile secretion with age. Other than the effect of aging on drug metabolism, liver reserve should be more than adequate even in the very old in the absence of disease.
Renal cortical mass also decreases by 20% to 25% with age, but the most prominent effect of aging is the loss of up to half of the
glomeruli by age 80.15 The decrease in the glomerular filtration rate of approximately 1 mL/min/yr after age 40 typically reduces renal excretion of drugs to a level where drug dosage adjustment becomes a progressively important consideration beginning at approximately age 60. Nevertheless, the degree of decline in glomerular filtration rate is highly variable and is likely to be much less than predicted in many individuals, especially those who avoid excessive dietary protein.16
glomeruli by age 80.15 The decrease in the glomerular filtration rate of approximately 1 mL/min/yr after age 40 typically reduces renal excretion of drugs to a level where drug dosage adjustment becomes a progressively important consideration beginning at approximately age 60. Nevertheless, the degree of decline in glomerular filtration rate is highly variable and is likely to be much less than predicted in many individuals, especially those who avoid excessive dietary protein.16
The aged kidney does not eliminate or retain sodium when necessary as effectively as that of a young adult.16 Part of the failure to conserve sodium when appropriate may be because of reduced aldosterone secretion. Similarly, the aged kidney does not retain or eliminate free water as rapidly as young kidneys when challenged by water deprivation or free water excess. Lastly, the sensation of thirst declines with age. In short, fluid and electrolyte homeostasis is more vulnerable in the older patient, particularly when an older patient suffers acute injury or disease and eating and drinking becomes more of a chore.
For the most part, functional endocrine decline does not interact with anesthetic management to any significant degree. However, aging is associated with decreased insulin secretion in response to a glucose load, and also increased insulin resistance, particularly in skeletal muscle.17 Thus, even healthy elderly patients may require insulin therapy more often perioperatively than young adults. Aging also results in decreases in testosterone, estrogen, and growth hormone production.18 The use of hormonal therapy to reduce sarcopenia, frailty in general, and cognitive decline and dementia is the subject of considerable current investigation, but has no current application to anesthetic management.
Central Nervous System Aging
By the time you’re eighty years old you’ve learned everything. You only have to remember it.
—George Burns
Brain mass begins to decrease slowly beginning at approximately age 50 and declines more rapidly later, such that an 80-year-old brain has typically lost 10% of its weight.19 Neurotransmitter functions suffer more significantly, including dopamine, serotonin, g-aminobutyric acid, and especially the acetylcholine system.20 The latter is especially important because of its connection to Alzheimer’s disease. Response times increase, and learning is more difficult, but vocabulary, “wisdom,” and past knowledge are better preserved.19 Nevertheless, of those individuals aged 85 and older, nearly half have significant cognitive impairment. In addition, some degree of atherosclerosis appears to be inevitable. Fortunately, and contrary to prior belief, the aged brain does make new neurons and is capable of forming new dendritic connections.21
Perhaps the best-known effect of brain aging as it applies to anesthesia is the approximately 6% decrease in MAC (minimum alveolar concentration) per decade after age 40.22 This effect of aging is relatively simple to deal with in the clinical arena. Much more difficult is the potential interaction of anesthesia, the stress of surgery, and a brain with minimal reserve. Age is a major risk factor for postoperative delirium and/or cognitive decline (see “Perioperative Complications”). The other pertinent brain aging phenomenon is pharmacodynamic.
Drug Pharmacology and Aging
I don’t do alcohol anymore – I get the same effect just standing up fast.
—Author Unknown
Drugs typically have a more pronounced effect in an older patient. The cause can be either pharmacodynamic, in which case the target organ (often the brain) is more sensitive to a given drug tissue level, or the cause can be pharmacokinetic, in which case a given dose of drug commonly produces higher blood levels in older patients.
Most intravenous anesthetic drugs are highly lipid soluble and so begin to enter tissue even before fully mixed in the blood. The rate of transfer depends on the rate of delivery (concentration times blood flow per gram of tissue), the concentration gradient of the drug between the blood and the tissue (obviously a high gradient initially), the ease with which the drug crosses the blood and tissue membranes, and the solubility of the drug in the tissue. Thus, the vessel-rich group (brain, heart, kidney, muscle) will acquire drug much more rapidly than the vessel-poor group (fat, bone). Protein binding may affect transfer, with drugs that are highly protein-bound having a lower free concentration and a slower rate of transfer.
Given the preceding, there are many ways for a drug bolus to have a more pronounced initial effect on older patients. During the drug redistribution phase the blood concentration typically is higher in older patients, partly because of a mildly contracted blood volume and partly because the reduction in muscle mass slows removal of the drug from blood. By keeping drug blood levels higher for a longer time, more drug will be driven into the other organs of the vessel-rich group such as brain (often the target organ) or heart. A prime example of this phenomenon is sodium pentothal, and to a lesser degree, propofol.23
Despite the fact that drugs typically have a greater effect on older patients, there is a general impression that bolus drugs take
longer to achieve that greater effect. It is not entirely clear why this is so. Slower circulation is sometimes hypothesized, but total blood flow to any organ does not appear to decrease beyond that expected from the decrease in organ mass. Another possibility is a slower rate of transfer into the target organ. Drug effects are the result of tissue, not plasma concentrations. Brain–plasma equilibration is not instantaneous, and for at least some drugs (e.g., remifentanil), the equilibration half-life is prolonged in older brains.24 Why crossing the blood–brain barrier should take longer with age is not understood.
longer to achieve that greater effect. It is not entirely clear why this is so. Slower circulation is sometimes hypothesized, but total blood flow to any organ does not appear to decrease beyond that expected from the decrease in organ mass. Another possibility is a slower rate of transfer into the target organ. Drug effects are the result of tissue, not plasma concentrations. Brain–plasma equilibration is not instantaneous, and for at least some drugs (e.g., remifentanil), the equilibration half-life is prolonged in older brains.24 Why crossing the blood–brain barrier should take longer with age is not understood.
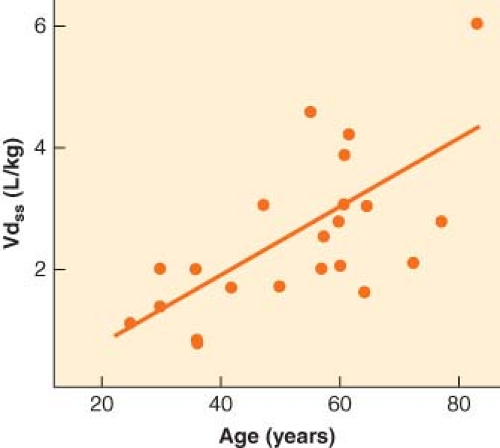
Ultimately, though, the drug will distribute throughout the body based on tissue mass and solubility. Because most intravenous drugs used in anesthesia are highly lipid-soluble, most of the drug will end up in fat. How completely the drug is dispersed out of the blood and into the tissue is reflected by Vdss, the drug’s volume of distribution at steady state. This variable is expressed as the liters of plasma that would be necessary to dilute the amount of drug administered down to the concentration observed in the plasma. As such, drugs that are very fat-soluble can have a value for Vdss that is several times greater than total body water. After the initial redistribution into vessel-rich group tissue, the drug will slowly diffuse back into the plasma as it continues to be absorbed into fat. In so doing, the target organ (e.g., brain) drug level will fall because the target organ is always in the vessel-rich group. Once a single therapeutic dose of a drug has fully distributed throughout the body, the blood and target organ drug levels are typically too low to have a meaningful clinical effect. However, very large doses, repeated doses, or infusions will eventually deliver enough drug to yield residual drug levels that produce therapeutic effects. At this point, the only way to decrease blood and target organ levels and eliminate the drug’s effects is through metabolism. The elimination or metabolic half-life of a drug in the blood equals the volume of distribution at steady state (Vdss) divided by the clearance, where clearance represents the amount of blood from which drug is eliminated per minute.
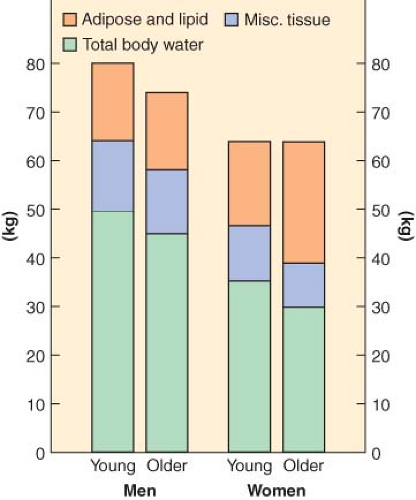
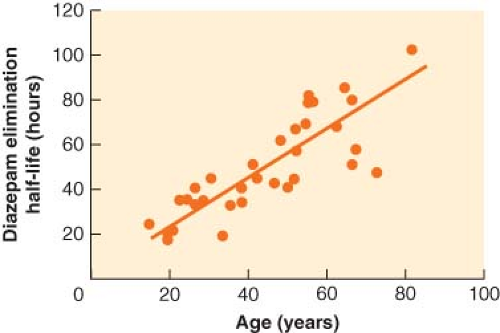
The most prominent and consistent pharmacokinetic effect of aging is a decrease in drug metabolism, typically due to both a decrease in clearance and an increase in Vdss (Fig. 33-3). The increase in Vdss with age is likely due to the increase in body fat. Clearance decreases with age for any drug metabolized by the liver or kidney. When drug metabolism is via the liver, decreased liver mass and blood flow will decrease clearance for both high and low extraction drugs. In addition, elderly patients are often on a host of chronic medications, a setup for drug interactions as well as for inhibition of drug metabolism. Drugs with primarily renal elimination will experience decreased metabolism because of reductions in glomerular filtration rate with aging. The net effect on drug metabolism is typically a doubling of the elimination half-life between old and young adults. However, with some drugs, the effect on half-life can be dramatic. In the case of diazepam, the half-life in hours is roughly equal to the patient’s age (Fig. 33-4).25 For a 72-year-old person, it would therefore require 3 days to metabolize half of a dose of diazepam. Such pharmacokinetics clearly illustrate why there is no place in modern medicine for the chronic use of diazepam and other drugs with similar half-lives when the desired effect is supposed to be transient (e.g., as a sleeping aid).
When dealing with infusions—or for that matter a series of bolus injections—the time it takes to decrease the blood and target organ drug levels to below the therapeutic threshold will depend on many factors. This is where the concept of the context-sensitive half-time proves useful; that is, the time necessary for a 50% (or any desired percent) decrease in plasma concentration following termination of an infusion. At one extreme, if the residual level produced by the cumulative drug administration is still very low, and only a modest decrease in blood level is necessary to reverse the drug effect, then the rapid redistribution of the most recently administered drug will lead to a rapid decrease in the blood level and termination of effect. At the other extreme, if there has been significant accumulation of drug in the body, and/or the maintenance blood level was high, then a long time may be required to decrease the drug levels enough to terminate the drug effect. As a general rule, the time to decrease the effect-site drug concentration is increased most dramatically by aging when a large percentage decrease in plasma level is necessary to dip below the therapeutic threshold.26
Review of the literature can yield a confusing picture when trying to sort out what pharmacologic variable is responsible for a given clinical effect. Fortunately, one does not need to know such details in order to use anesthetic drugs in an intelligent fashion with older patients. Table 33-1 summarizes some of this information for many of the common anesthetic drugs.26,27,28,29 The effect of aging on sedative–hypnotic agents variably involves both pharmacodynamic and pharmacokinetic changes (Table 33-1).
For the opioids, the older brain appears to be more sensitive than that of young adults, whereas the pharmacokinetics of opioids are largely unaffected by age.
For the opioids, the older brain appears to be more sensitive than that of young adults, whereas the pharmacokinetics of opioids are largely unaffected by age.
Table 33-1. Effect of Age on Drug Dosing | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Despite the loss of muscle and motor neurons with age, muscle relaxants do not appear to be more potent in the older patient when steady-state blood levels for a given level of paralysis are compared. Muscle relaxants often have a decreased initial volume of distribution, but this pharmacokinetic change does not seem to translate into smaller doses. For drugs eliminated by the liver or kidney, and where the effect of a bolus is eliminated primarily by redistribution, multiple doses will result in drug accumulation, and each subsequent dose will have a more prolonged effect. This phenomenon will be exaggerated in elderly patients because of decreased metabolic elimination, and will be most prominent with the long-acting agents. Given the risk of residual neuromuscular blockade with long-acting drugs such as pancuronium, coupled with the muscle and nervous system changes of aging that increase the risk of ventilatory failure or aspiration postoperatively, it can be argued that long-acting neuromuscular blocking agents should be used very carefully in an older patient, if at all.
Cardiovascular Aging
A man is as old as his arteries.
—Thomas Sydenham
Virtually all components of the cardiovascular system are affected by the aging process. The major changes include (1) decreased response to β-receptor stimulation; (2) stiffening of the myocardium, arteries, and veins; (3) changes in the autonomic nervous system with increased sympathetic activity and decreased parasympathetic activity; (4) conduction system changes; and (5) defective ischemic preconditioning. Although atherosclerosis appears to affect everyone by virtue of the fact that the mechanisms of aging contribute to the development of atherosclerosis, it is not clear that it inevitably leads to functional impairment or disease.
With age, increased sympathetic activity is present at rest and there is typically an exaggerated response to stimuli that increase sympathetic activity.30 Although there is some evidence of decreased responsiveness of α-receptors with age, it apparently is not enough to prevent changes in vascular resistance from making a significant contribution to the lability in blood pressure observed during anesthesia or contribute to the decrease in blood pressure when anesthesia removes that sympathetic tone.31,32
The efficacy of baroreflex control of blood pressure decreases with age.33 The mechanism is primarily a decrease in the heart rate response and not a decrease in the baroreflex control of vascular tone. The decreased heart rate response to changes in blood pressure is in part due to lesser vagal tone at rest but the major mechanism is a decrease in the cardiac response to β-receptor stimulation.32 The mechanism does not appear to be a downregulation of β-receptors on the heart, but a defect in the intracellular coupling. Both heart rate and contractility increase less in response to endogenous release or exogenous administration of catecholamines. The increase in heart rate with exercise is therefore also diminished, as is maximal heart rate (often quoted as 220 minus age), and the decrement contributes to the decreased exertional capacity with age, even in trained individuals. The decrease in resting vagal tone may limit the increase in heart rate after administration of atropine or glycopyrrolate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree