Native heart
OHTx recipient
Innvervation
Autonomic and sensory innervation intact
Denervated initially—partial reinnervation time course. The exact time course is unclear at present
Resting heart rate
60–80 beats/min
90–110 beats/min
EKG findings
Normal
Commonly two P-waves
Arrhythmias
Not common
Very common
Response to stress
Intact reflex pathways
Loss of baroreceptor reflex, inability to increase heart rate with hypotension/hypovolemia
Transplanted hearts are known to have an elevated resting heart rate of approximately 90–110 beats/min [3, 7, 8]. This generally equates to a resting atrial rate that is 14–25 beats/min higher than the resting atrial rate for age- and sex-matched controls compared with non-transplanted hearts [9]. The elevation in heart rate in the transplant recipient is owed to the absence of vagal tone leaving the heart rate dependent upon the intrinsic rate of depolarization of the donor SA node [3].
Another significant difference between the transplanted heart and the normal heart is found in the response to physiologic stressors such as hypovolemia and hypotension. The normal heart has neural mechanisms in place that permit heart rate and cardiac output increases in response to stress, but the transplanted heart is denervated and lacks this ability [3, 8]. Early in the stress response, the heart rate and cardiac output of the transplanted heart are relatively fixed. The Frank-Starling mechanism of the transplanted heart does remain intact; consequently, increases in cardiac output are dependent upon increases in venous return leading to an increased LVEDV. For this reason, transplanted hearts are often referred to as “preload dependent” [3, 8]. Later into the course of the stress response, there is an increase in circulating catecholamines, but this process takes 5–6 min. Remembering that the transplanted heart has intact alpha and beta-adrenoreceptors, increasing circulating catecholamines yield an increase in chronotropy and inotropy [3].
Reinnervation of the transplanted heart continues to be a controversial topic. Recent studies show that between 33 and 41 % of patients exhibit a partially normalized response to exercise within the first year [10, 11]. This implies that partial reinnervation may occur very early in the post-transplant period. Reinnervation appears to be a continuous process that is very heterogeneous in nature [12]. Some patients will exhibit complete reinnervation with normalization of cardiac reflex pathways, but this does not typically occur until at least 15 years following transplantation [6, 12].
Cardiac denervation , not unexpectedly, alters the pharmacology of many drugs used in the perioperative period, which must be taken into account. It has been reported that the density of catecholamine receptors in the transplanted heart is unchanged compared to the native heart. Owing to this, direct-acting drugs such as epinephrine and norepinephrine will remain effective in increasing heart rate and contractility in OHTx recipients [7]. It was long hypothesized that drugs such as anticholinergics (glycopyrrolate and atropine), muscle relaxants (pancuronium), and acetylcholinesterase inhibitors (neostigmine, edrophonium, pyridostigmine, and physostigmine) had zero impact on the denervated heart, but this topic should be approached with caution [13, 14]. It has been clearly shown that some component of sympathetic and parasympathetic innervation is established in most patients after heart transplantation leading to these patients having an unpredictable response to indirect-acting drugs [15]. In lieu of this fact, there are numerous reports of asystole following the administration of neostigmine for the reversal of neuromuscular blockade in transplant recipients [15–18]. Medication administration must be carefully thought out in this patient population and avoidance of neuromuscular blocking drugs may be best if at all possible.
Complications Following Heart Transplantation/Post-transplant Morbidities
Complications following heart transplantation can be separated into problems that arise in the immediate postoperative period and those that occur on a more long-term basis. Potentially catastrophic issues that develop shortly after surgery include right ventricular dysfunction, acute renal failure (ARF), and acute graft rejection. The years after transplant are also fraught with morbidities such as coronary vasculopathy, hypertension, chronic renal insufficiency, and malignancy. Anesthesiologists must be aware of these common disease processes to provide optimal patient care. Table 18.2 shows a detailed incidence of many of the post-transplant complications [19].
Table 18.2
Post-transplant morbidities : Incidence of common issues 10 years following heart transplantation
Disease process | Incidence at 10 years (%) |
|---|---|
Renal insufficiency (Cr > 2.5 mg/dL) | 14 |
Hypertension | 97 |
Diabetes mellitus | 39 |
Coronary artery vasculopathya | 52 |
Malignancy | 34 |
Right Ventricular Dysfunction
Pre-existing pulmonary hypertension confers an increased risk of acute right ventricular failure following cardiac transplantation. Given this concern, most transplant centers view elevated pulmonary vascular resistance as a contraindication to heart transplantation [20]. Right ventricular dysfunction may also be secondary to poor preservation of the graft prior to transplantation [21]. Regardless of the etiology of right ventricular dysfunction, the management is similar to this issue occurring in the native heart. First, there should be rapid evaluation of oxygenation and ventilation. Secondly, pharmacotherapy may be extremely beneficial including drugs such as inhaled nitric oxide, inhaled and intravenous prostaglandin E1, milrinone, dobutamine, and epinephrine depending on the clinical scenario.
Acute Renal Failure
Oliguria and ARF after heart transplant surgery often develop as a result of cardiopulmonary bypass, low flow states, and cyclosporine induction therapy [21]. Prior to a 2010 study by Gude and colleagues, there was little incidence data on the topic of immediate post-transplantation renal insufficiency. This study retrospectively evaluated 585 heart transplant recipients and found a 25 % incidence of ARF. The primary risk factors associated with the development of ARF included intravenous cyclosporine administration, increased donor age, and increased recipient age. While patients who progressed to chronic renal insufficiency had an increase in mortality, it did not appear that the development of ARF in the immediate postoperative period is predictive of the subsequent need for dialysis or renal transplantation in this patient population [22].
Donor Graft Rejection
Prevention of graft rejection requires a delicate balance of the immunosuppression regimen with too much immunosuppression increasing infectious risks, but too little risking organ rejection [21]. Rejection episodes most commonly occur within the first 3 months following heart transplant surgery with a peak incidence near 6 weeks after transplantation [3]. According to the International Society for Heart and Lung Transplantation (ISHLT), the incidence of treated acute allograft rejection ranges from 21 to 30 % depending on the immunosuppression protocol followed [19]. It remains true that acute rejection is exceedingly unlikely after the first year but must be considered in any patient who is not taking their immunosuppressive regimen as indicated [6].
The gold standard of diagnosing a rejection episode hinges on the endomyocardial biopsy [21]. Common patient symptoms during an episode of rejection include arrhythmias, fever, fatigue, weight gain, peripheral edema, shortness of breath, and bradycardia [6]. A low level of suspicion must always remain to work up a potential rejection episode as this can prove to be a fatal event. Treatment of the acute rejection episode generally entails increasing the immnosuppression regimen for acute rejection, IV immunoglobulins and plasmapheresis for antibody-mediated rejection, and potentially temporary mechanical support depending on the severity of the presentation [6].
Coronary Artery Vasculopathy
Coronary artery vasculopathy (CAV) is one of the leading causes of mortality following OHTx according to the ISHLT [23, 24]. CAV currently accounts for 10–14 % of deaths more than 1 year post-transplant [23]. Medical management and advances in immunosuppression have greatly improved survival after OHTx in recent years, but the CAV incidence remains unchanged. The current estimates for CAV among heart transplant recipients are 20 %, 30 %, and 45 % at 3, 5, and 8 years post-transplant, respectively [23].
It is likely that some coronary arterial disease is transplanted with the donor organ, but CAV frequently occurs in recipient organs that did not have any pre-existing coronary disease. Certain risk factors for the development of CAV have been clearly identified including recipient age, pre-existing ischemic heart disease, cyclosporine immunosuppression versus tacrolimus, and even use of a pre-transplant ventricular assist device for the treatment of heart failure [23]. Current treatment options used with an attempt to decrease morbidity and mortality from CAV include diltiazem and pravastatin or simvastatin. These have been shown to reduce, but not prevent, CAV development [25]. Aggressive ongoing research exists with an attempt to find a cure or more effective treatment for CAV. At this time, mTOR inhibitors are the most promising drugs to reduce CAV, but a survival benefit has not been shown to date and side effects can be troublesome [24].
Hypertension
Hypertension following OHTx is exceedingly common and typically due to cyclosporine therapy [3, 21, 26, 27]. Prior to the usage of cyclosporine as part of the immunosuppression regimen, hypertension after OHTx was only seen in approximately 20 % of patients [28]. More recently, the documented rate of post-transplant hypertension is greater than 90 % with one study citing a 97 % incidence at 10 years [19, 28, 29]. Patients at increased risk of developing early post-transplant hypertension include patients of advanced age and those with pre-existing hypertension. Pharmacotherapy is typically able to achieve sufficient blood pressure control and many patients can be controlled with single-drug therapy [30].
Chronic Renal Insufficiency
Common associations with chronic renal insufficiency in the OHTx recipient include the chronic low flow state associated with advanced heart failure leading to compromised renal arterial flow, cardiopulmonary bypass exposure, and the immunosuppression regimen in the years following transplantation. Immunosuppression regimens are credited with marked improvements in survival following organ transplantation, but these drugs do not come without a cost. In particular, calcineurin inhibitors such as cyclosporine are well known for causing nephrotoxicity and renal failure [31–33]. Severe renal dysfunction, defined as a serum creatinine of greater than 2.5 mg/dl, is extremely common following heart transplant with numbers approaching 15 % by 10 years [19].
Recent studies have attempted to define the risk factors leading to severe renal dysfunction in the years following OHTx. Common risk factors include advanced age, recipient diabetes mellitus, and elevated preoperative serum creatinine [19, 34, 35]. Due to the known association of OHTx and renal failure, it is extremely important to avoid the co-administration of other nephrotoxic medications in this patient population.
Arrhythmias
Cardiac dysrhythmias are common in the cardiac transplanted recipient due to denervation, rejection, and increased endogenous catecholamine concentrations. The most common indication for permanent pacemaker (PPM) implantation after OHTx remains significant bradycardia that is typically secondary to sinus node dysfunction [3, 36, 37]. Recent studies show that the surgical technique is a strong predictor of the need for PPM with a biatrial technique significantly increasing the risk [37, 38]. In the past, it was believed that PPM was uncommon after OHTx, but more recent literature reveals that 10–20 % of patients will ultimately require pacemaker insertion [38, 39]. A topic that requires further investigation is the fact that there appears to be a decrease in long-term mortality in patients who have pacemakers placed in the perioperative period following transplantation [38].
Malignancy
It is well known that patients receiving solid organ transplants and immunosuppression are at a greater risk of malignancy than the general population. The most common types of cancers following OHTx are skin cancers with greater than 15 % of recipients ultimately being affected [19, 40, 41]. The largest database from the International Society for Heart and Lung Transplantation reports that by 10 years post-transplant only 66 % of patients will be free of any malignancy [19]. More serious diseases such as lymphoproliferative disease are not uncommon in this patient population with 1–2 % of patients impacted within the first 5 years after surgery [42].
Common Procedures After Orthotopic Heart Transplantation
It is well described that a substantial number of patients will present to the operating room for general surgical conditions following OHTx [2, 5, 43]. The high rate of general surgery in this patient population is often attributed to the low flow state preoperatively, intraoperative cardiopulmonary bypass, and the use of immunosuppression in the postoperative period [2]. Diagnosing general surgical conditions in the heart transplant recipient can be challenging due to the fact that immunosuppressive drugs may mask the typical presenting symptoms and hasten the progression of disease.
The immediate post-transplant period represents the most likely time for an OHTx patient to require general surgery [2]. Surgeries in this time period are occasionally due to surgical complications, but may also include procedures such as exploratory laparotomy and bowel resections [44]. The requirement for general surgery within 30 days following OHTx confers a substantial increase in mortality partially owing to the fact that diagnosis is difficult in this period and immunsuppression makes recovery challenging [2, 44]. As you move further away from the time of transplant surgery, the most common general surgical conditions patient seek treatment for remain intra-abdominal pathology, such as cholecystectomy, hernia repair, and appendectomy [2, 5].
Preoperative Evaluation
The preoperative assessment of any transplant recipient must include a thorough assessment of graft function, infection, rejection, and the function of other organs that may be compromised as a result of chronic immunosuppressive therapy [7]. A dedicated transplant team closely monitors transplant recipients and it is prudent to discuss patient management with this team prior to performing elective noncardiac surgery. The transplant team is able to divulge important information regarding the immunosuppressive regimen, episodes of rejection, status of the transplanted organ, and complications that have arisen since the time of transplant. In the setting of more emergent surgery, the anesthesiologist must then rely on patient history and laboratory/other data that is available to best manage the OHTx recipient.
Necessary preoperative testing for the OHTx includes a current electrocardiogram, echocardiogram, and laboratory assessment [6]. It is best to be able to compare the current electrocardiogram with prior electrocardiograms to evaluate for any new findings. Preoperative echocardiography can be extremely helpful and yields a rapid way to evaluate ventricular function. Echocardiography may also shed light on any new valvulopathy since the time of transplant. In regard to laboratory evaluation, particular attention should be paid to markers of infection as well as renal indices given the high incidence of renal insufficiency following heart transplantation. The remainder of the preoperative examination should be no different between the OHTx recipient and any other patient.
Anesthesia Management and Considerations
Proper anesthesia management requires a detailed understanding of the physiology of the transplanted heart and the comorbidities associated with OHTx. After a comprehensive preoperative examination, standard premedication should be given as in non-transplant patients [7]. As in most cases, the type of anesthesia utilized is dictated by the surgical requirements. General, neuraxial, and regional anesthesia as well as monitored anesthesia care have all been safely used in this patient population [7]. A valid concern with the use of neuraxial anesthesia is that acutely decreasing preload may lead to severe hypotension in a patient who is “preload dependent.” Intravascular volume administration prior to neuraxial block may help to augment the severity of hypotension, but some recommend avoiding neuraxial blocks in OHTx recipients due to the unpredictability of the hemodynamic response.
Intraoperative monitoring with standard ASA monitors may be all that is required for patients following OHTx [45]. If invasive monitors are planned in the setting of predicted large fluid shifts, one must weigh the risks of infection versus the benefits of invasive monitoring techniques. Strict care must be taken to ensure that complete aseptic technique is used with the insertion of invasive monitors due to the increased risk of infection in patients on immunosuppressive regimens [7, 46]. As opposed to a pulmonary artery catheter, transesophageal echocardiography may be a more helpful monitor to evaluate volume status and cardiac contractility with a decreased risk of infection [7].
Medication administration by the anesthesia provider must also be carefully considered. As mentioned previously, indirect-acting drugs such as anticholinergics may be of no benefit in increasing heart rate and contractility. The transplanted organ does maintain a normal density of intrinsic adrenergic receptors and direct-acting drugs such as epinephrine and norepinephrine are often the most useful in treating hypotension. Intravenous fluid boluses should also be considered early in the management of hypotension. The muscle relaxant used to maintain balanced anesthesia should be chosen with caution as well; cis-atracurium is often an excellent choice due to the fact that elimination is not affected by either renal or hepatic dysfunction. The choice of reversal of muscle relaxation must also be taken seriously because there are numerous reports of neostigmine-induced asystole following OHTx. Some providers avoid the use of neuromuscular blocking drugs entirely to avoid this described complication.
Noncardiac Surgery Following Lung Transplantation
Introduction
End-stage lung disease caused by obstructive, restrictive, and pulmonary vascular disease is often treated with either single- (SLTx) or double-lung transplantation (DLTx). Lung transplantation (LTx) is becoming increasingly common to improve patient quality of life as well as extend survival. Graft survival and patient outcomes may be impacted by both immediate- and long-term complications that are well described following LTx surgery. Immediate concerns such as infection or graft rejection and long-term issues such as bronchiolitis obliterans, cancer, and systemic disease may all influence the final outcome [47].
As with most forms of organ transplantation, survival following LTx surgery continues to improve. The most recent statistics published by the Organ Procurement and Transplantation Network show the 1- and 5-year survival to be at 82 % and nearly 50 %, respectively [1]. Data published by the ISHLT shows that survival is influenced by both the type of disease requiring transplant and the type of transplant performed with patients receiving a DLTx living longer than SLTx recipients [48]. While survival advances, the number of organ transplanted is also on the rise with nearly 1800 LTx procedures occurring in the US annually [1]. With improved life expectancy and increasing numbers of transplanted organs, more patients following LTx are presenting to the operating than ever before for noncardiac surgery.
Physiology of the Transplanted Lung
Major physiologic changes occur in the transplanted lung secondary to the disruption of innervation, lymphatics, and bronchial circulation during lung procurement and insertion. The extent of physiologic change depends upon the type of transplant performed (single- vs. double-lung transplant), surgical technique, and the indication for transplantation. It is important for the anesthesiologist to be familiar with post-transplant physiology to optimally manage LTx recipients in the postoperative period and in the years following transplant.
Loss of the cough reflex distal to the site of the bronchial anastomosis is possibly the most devastating complication of denervation [49]. This occurs because the surgical procedure involves transection of the vagal nerve with resultant sensory and autonomic airway denervation distal to the site of airway anastomosis [50]. The current surgical technique attempts to preserve the carina at all cost in an attempt to maintain a normal reflex pathway in the proximal airway. The concern with losing the cough reflex comes from data indicating an increased risk of premature death as a result of infectious complications, a major cause of post-transplant morbidity and mortality [51]. While it was previously thought that loss of the cough reflex distal to the anastomosis was a permanent issue, newer literature suggests that this may not be true and that reflex pathways may be restored within 6–12 months following postoperatively [49, 50, 52].
Other physiologic consequences of denervation include impaired mucociliary transport and loss of baroreceptor input from the medulla to the lung [53–55]. Despite these changes, respiratory rate and rhythm appear to be unchanged following double-lung transplantation [45]. It also appears that airway tone, which is mediated primarily by the parasympathetic nervous system (PNS), is not adversely affected. This is due to the fact that muscarinic receptors on transplanted lung/lungs remain intact and responsive to efferent signals sent by the PNS. The airway should remain responsive to the effects of beta-2 agonists such as albuterol [47].
Pulmonary blood flow following LTx depends on whether the patient receives an SLTx vs. DLTx procedure. DLTx recipient lungs have normal pulmonary blood flow, but SLTx patients have 60–70 % of the perfusion and ventilation going toward the transplanted lung [56]. Regardless of the type of transplant that is performed, it appears that hypoxic pulmonary vasoconstriction is preserved in LTx recipients [57]. One remaining concern in the LTx recipient is that these patients may be extremely sensitive to fluid shifts and fluid overload. Lymphatic interruption is a known side effect of the surgical procedure and small volume challenges may potentially cause pulmonary edema in LTx patients [58]. The lymphatic channels are eventually restored, but the timing and extent of reformation remain unclear [59, 60].
Complications Following Lung Transplantation/Post-transplant Morbidities
Recipients of transplanted lungs are at risk for numerous adverse events linked to the disease necessitating transplantation, the surgical procedure, and the immunosuppression regimens postoperatively. Major concerns in the immediate period following surgery include graft failure, bleeding, and infection. Moving further away from the time of transplantation, common morbidities seen are typically elicited or exacerbated by the immunosuppression regimen chosen and these are highlighted in Table 18.3. Two topics deserving further discussion in the patient following LTx are bronchiolitis obliterans and infection.
Table 18.3
Post-transplant morbidities : Incidence of common issues at 1 and 5 years following lung transplantation
Disease | Incidence at 1 year (%) | Incidence at 5 years (%) |
|---|---|---|
Bronchiolitis obliterans | 9.5 | 38.9 |
Hypertension | 52.0 | 82.9 |
Creatinine <2.5 mg/dl | 16.5 | 36.7 |
Dialysis dependent | 1.7 | 3.2 |
Diabetes mellitus | 25.5 | 40.5 |
Hyperlipidemia | 25.0 | 57.9 |
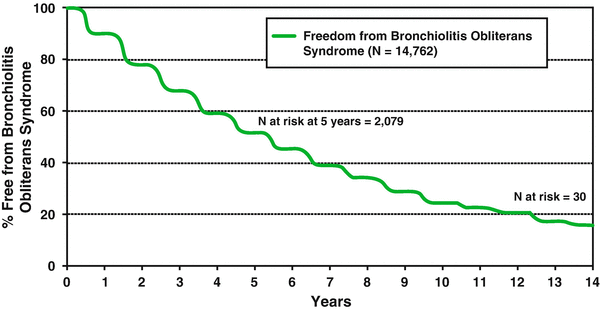
Bronchiolitis Obliterans
Although short -term survival following LTx continues to improve, bronchiolitis obliterans (BO) remains a major threat to advances in long-term survival [61, 62]. The most recent report released from the ISHLT in 2012 describes the incidence of BO to be 48 % by 5 years and 76 % by 10 years post-transplant—see Fig. 18.1 for details. The diagnosis of BO confers a very high probability of mortality and survival following diagnosis is only 30–40 % at 5 years [63].