Anesthesia and Obesity
Brenda A. Bucklin
Ana Fernandez-Bustamante
Key Points
Related Matter
Sequelae of Obesity
Sleep Apnea
Definition and Epidemiology
Introduction
The World Health Organization defines obesity as a condition with excess body fat to the extent that health and well-being are adversely affected.1 Obesity is reaching epidemic proportions worldwide. For the first time in history, recent estimates suggest that the number of obese individuals now exceeds the number of underweight individuals. About one-third of Americans (33.8%) are currently obese.2 The prevalence of obesity in the United States is unevenly distributed geographically, by race and ethnicity and by socioeconomic status. The overall trend is, however, to keep increasing. The prevalence of obesity is predicted to reach about 50% by 2030.3 The Centers for Disease Control and Prevention (CDC) monitor the epidemiology of obesity and publish periodically updated data at http://www.cdc.gov/obesity/.
Obesity-related conditions including cardiovascular accidents, insulin- and noninsulin-dependent diabetes, and some types of cancer are the leading causes of death in this population.4 Although there has been an exponential increase in the number of bariatric procedures performed, obese and morbidly obese patients undergo all types of surgical procedures. Surgery in this patient population is considered high-risk but careful planning, preoperative risk assessment, proper anesthetic management, strict venothrombotic event prevention, and effective postoperative pain control will all help to reduce the risk. With appropriate perioperative management, obese surgical patients can achieve safe and effective surgical outcomes.
The definition of obesity includes the presence of excessive body weight for the patient’s age, gender, and height, and is often based on the following concepts. Ideal body weight (IBW) is a concept originated by life insurance companies by referencing height–weight tables. It is the weight associated with the lowest mortality rate for a given height and gender and can be estimated using Broca’s index:
where x is 100 for adult males and 105 for adult females.
Predicted body weight (PBW) is a concept similar to IBW, and is more commonly used in the medical literature. PBW is usually calculated with the following formulas in adults5:
Lean body weight (LBW) is the total body weight (TBW) minus the adipose tissue. It is a combination of body cell mass, extracellular water, and nonfat connective tissue. It approximates 80% and 75% of TBW for males and females, respectively, although more accurate formulas have been proposed.6,7 In morbidly obese patients, increasing the IBW by 20% to 30% gives an estimate of LBW. In nonobese and nonmuscular individuals, TBW approximates IBW.8
In clinical practice it is common to utilize the Body mass index (BMI), also called Quetelet’s index, to estimate the degree of obesity. The BMI is determined using the patient’s measured weight (in kilograms) and height (in meters) and is calculated as follows:
Obesity is defined as having a BMI ≥30 kg/m2. Obesity is further classified according to systemic disease risk (Table 44-1). Morbid obesity, defined as a BMI ≥40 kg/m2, can also be further classified into super obesity (BMI ≥50 kg/m2) and super-super obesity (BMI ≥60 kg/m2).9 BMI differentiates obese from nonobese adults and it estimates body fat because it adjusts for height while strongly correlating with body weight; however, it cannot distinguish between overweight and overfat, as heavily muscled individuals can be easily classified as overweight using BMI. Therefore, other factors such as age, fat content, and distribution (i.e., waist circumference and waist-to-hip ratio) should be taken into consideration, along with other health risk predictors that use the concept of BMI.10
The anatomic distribution of body fat has associated pathophysiologic implications.11,12 In android (central) obesity, adipose tissue is located predominantly in the upper body (truncal distribution) and is associated with increased oxygen consumption and an increased incidence of cardiovascular disease. Visceral fat is particularly associated with cardiovascular disease and left ventricular dysfunction. In gynecoid (peripheral) obesity, adipose tissue is located predominantly in the hips, buttocks, and thighs.
This fat is less metabolically active so it is less closely associated with cardiovascular disease. Body circumference indices such as waist circumference, waist-to-height ratio, and waist-to-hip ratio help to classify these patterns of obesity (e.g., android vs. gynecoid obesity) and correlate with mortality and the risk for developing obesity-related diseases. Waist circumference correlates with abdominal fat and is an independent risk predictor of disease.
This fat is less metabolically active so it is less closely associated with cardiovascular disease. Body circumference indices such as waist circumference, waist-to-height ratio, and waist-to-hip ratio help to classify these patterns of obesity (e.g., android vs. gynecoid obesity) and correlate with mortality and the risk for developing obesity-related diseases. Waist circumference correlates with abdominal fat and is an independent risk predictor of disease.
Table 44-1. Classification of Obesity and Systemic Disease Risk According to Waist Circumference | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Management of Obesity
Medical Therapy
The indications for pharmacologic treatment include a BMI ≥30 kg/m2 or a BMI between 27 and 29.9 kg/m2 in conjunction with an obesity-related medical complication. Lifestyle counseling is still the most effective tool for long-term weight loss, but it can be combined with the use of medications.13,14 Medications used to treat obesity are formulated to reduce energy intake, increase energy utilization, or decrease absorption of nutrients. The only currently FDA-approved antiobesity medications are phentermine and orlistat.15 Phentermine (Adipex-P) is a sympathomimetic drug that decreases appetite. Although it is only approved for 3 months use, it can induce tachycardia, palpitations, hypertension, as well as dependence, abuse, and withdrawal symptoms. It is no longer combined with fenfluramine (Phen-Fen) due to concerns of pulmonary hypertension and valvular heart disease,16 but is being explored in combination with topiramate (Topamax).17 This combination often causes dry mouth, paresthesias, constipation, insomnia, and dizziness. Orlistat (OTC Alli, prescribed Xenical) or tetrahydrolipstatin, blocks the absorption of dietary fat by inhibiting lipases in the gastrointestinal tract. It leads to weight loss and to improvement of blood pressure, fasting blood glucose levels, and lipid profile.18 Fat malabsorption causes common complaints of oily spotting, liquid stools, fecal urgency, flatulence, and abdominal cramping. Chronic use of orlistat may result in fat-soluble vitamin deficiency. A prolonged prothrombin time with a normal partial thromboplastin time during orlistat treatment may reflect vitamin K deficiency and this coagulopathy should be corrected 6 to 24 hours before elective surgery.19
A variety of over the counter preparations, plant extracts, or herbs are often used by patients to combat obesity. Substances found in these preparations that are allegedly thought to promote fat loss include pancreatic lipase inhibitors (caffeine, green or black tea), appetite suppressants (hoodia, Korean ginseng, ephedra, sunflower oil), stimulants of energy expenditure (acai berry, caffeine), and regulators of lipid metabolism (soybean, fish oil, oolong tea, caffeine).20 The American Society of Anesthesiologists (ASA) warns patients to tell their anesthesiologists about medications they are taking, including vitamins, herbs and other supplements, since these products may interfere with anesthesia or cause complications during surgery.21
Surgical Therapy (Bariatric Surgery)
Bariatric surgery is currently the most effective treatment for morbid (class III) obesity. Several guidelines exist for determining patient eligibility for bariatric surgery. Most agree that acceptable patients for surgery are those with a BMI >40 kg/m2 or BMI >35 kg/m2 and/or those patients with obesity-related comorbidities not controlled with medical therapy.22 Procedures are grouped into three classifications. Malabsorptive procedures include jejunoileal bypass and biliopancreatic diversion, and are rarely used nowadays. Restrictive procedures include vertical-banded gastroplasty and adjustable gastric banding. Combined procedures include Roux-en-Y gastric bypass (RYGB), which combines gastric restriction with a minimal degree of malabsorption. RYGB, adjustable gastric banding, and vertical-banded gastroplasty can all be performed laparoscopically. Laparoscopic bariatric surgery is associated with less postoperative pain, lower morbidity, faster recovery, and less “third-spacing” of fluid.23 RYGB is the most effective bariatric procedure to produce safe short- and long-term weight loss in severely obese patients. With RYGB, patients lose an average of 50% to 60% excess body weight and show a BMI decrease of approximately 10 kg/m2 during the first 12 to 24 postoperative months. Type II diabetes resolves in a majority of patients. Laparoscopic adjustable gastric banding (LAGB) is a restrictive gastric operation that utilizes an adjustable inflatable band to alter stomach capacity for individual weight loss needs. Vertical-banded or sleeve gastroplasty also restricts food intake.
Less invasive bariatric techniques are being developed. An implantable gastric stimulator (IGS) is placed laparoscopically and emits electrical impulses to stimulate the gastric smooth muscle to stop peristalsis so that the patient feels full. The IGS can be adversely affected by defibrillation, electrocautery, lithotripsy, magnetic resonance imaging, and therapeutic radiation. Intragastric balloons and prostheses, at different experimental stages, are placed endoscopically as a temporary measure to increase satiety.24 Adequate control of postoperative nausea and vomiting is critical to avoid possible lead and balloon dislodgement. Although apparently simple and safe as bariatric procedures, their efficacy for weight loss is still questioned.22,25
Pathophysiology
Respiratory System
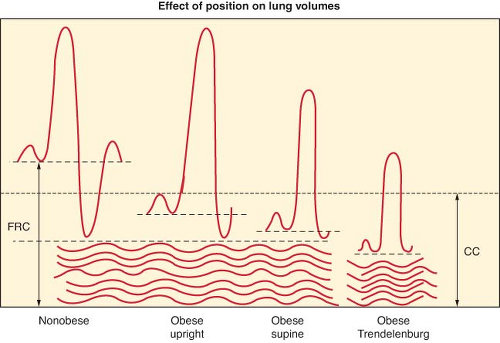
Fat accumulation on the thorax and abdomen decreases chest wall and lung compliance. Decreased lung compliance is partially explained by increased pulmonary blood volume related to an overall increase in blood volume. Increased elastic resistance and decreased compliance of the chest wall are further reduced while supine, leading to shallow and rapid breathing, an increased work of breathing, and limited maximum ventilatory capacity. Respiratory muscle efficiency is below normal in obese individuals. Decreased pulmonary compliance leads to decreased functional residual capacity (FRC), vital capacity, and total lung capacity. Reduction in FRC is primarily a result of reduced expiratory reserve volume (ERV), but the relationship between FRC and closing capacity, the volume at which small airways begin to close, is adversely affected (Fig. 44-1). Decreases in FRC and ERV are the most commonly reported abnormalities of pulmonary function in obese patients.26,27 Residual volume and closing capacity are unchanged. Reduced FRC (due to decreased ERV) can result in lung volumes below closing capacity in the course of normal tidal ventilation, leading to small airway closure, ventilation–perfusion mismatch, right-to-left shunting, and arterial hypoxemia. Anesthesia and supine positioning worsen this situation such that up to a 50% reduction in FRC occurs in the obese anesthetized patient compared with 20% in the nonobese individual. Forced expiratory volume in 1 second and forced vital capacity are usually within normal limits. ERV is the most sensitive indicator of the effect of obesity on pulmonary function.
Table 44-2. Anesthetic Implications of Obesity | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Obesity increases oxygen consumption and carbon dioxide production even at rest. This is because of the metabolic activity of excess fat and the increased workload on supportive tissues. The body attempts to meet these metabolic demands by increasing both cardiac output and alveolar ventilation. Basal metabolic activity is usually within normal limits in relationship to body surface area and normocapnia is usually maintained by an increase in minute ventilation. This requires increased oxygen consumption because most obese patients retain their normal response to hypoxemia and hypercapnia. Arterial oxygen tension in morbidly obese patients’ breathing room air is lower than that predicted for similarly aged nonobese subjects in both sitting and supine positions. Chronic hypoxemia may lead to polycythemia, pulmonary hypertension and cor pulmonale.
The “gold standard” diagnostic test for OSA is overnight polysomnography (OPS). The inconvenience, time, and expense of OPS lead to an unknown fraction of obese patients with suspicious but no formal diagnosis of OSA.28 Suggestive signs to look for during the preoperative visit include witnessed episodes of apnea during sleep, BMI ≥35, neck circumference ≥16 in (≥40 cm), hyperinsulinemia, and elevated glycosylated hemoglobin. Symptoms of snoring, frequent arousals during sleep and daytime sleepiness, impaired concentration, memory problems, and morning headaches are common but not predictive.29,30 A thorough preoperative evaluation for possible OSA is recommended long enough before elective surgery to allow preparation of a perioperative management plan.31 Preoperative initiation of continuous positive airway pressure (CPAP), especially in severe OSA cases, should be considered.31 Patients with confirmed or suspected OSA are at high risk of presenting with a difficult airway and postoperative pulmonary complications, and should be managed accordingly.32,33
The obesity hypoventilation syndrome (OHS) or Pickwickian syndrome may result from long-term OSA and is seen in 5% to 10% of morbidly obese patients. The OHS is a combination of obesity and chronic hypoventilation that ultimately results in pulmonary hypertension and cor pulmonale.34 The presence of both obesity (BMI >30 kg/m2) and awake arterial hypercapnia (PaCO2 >45 mm Hg) in the absence of known causes of hypoventilation supports the diagnosis. Prolonged OSA also alters the control of breathing, leading to CNS-mediated apneic events. This increases reliance on hypoxic drive for ventilation. The main ventilatory impairment of OHS is alveolar hypoventilation independent of intrinsic lung disease. Other characteristics of OHS include daytime hypersomnolence, hypercapnia, hypoxemia, and polycythemia. Right ventricular failure eventually ensues. These patients also have an increased sensitivity to the respiratory depressant effects of general anesthetics.
Cardiovascular and Hematologic Systems
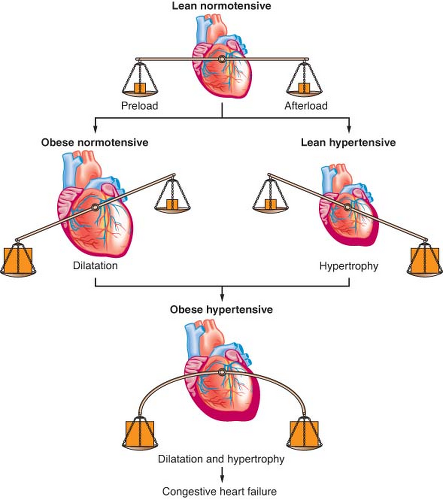
Total blood volume is increased in the obese individual; but on a volume-to-weight basis, it is less than in nonobese individuals (50 mL/kg compared with 70 mL/kg). Most of this extra volume is distributed in the adipose tissue. Renal and splanchnic blood flows are increased. Cardiac output increases with increasing weight by as much as 20 to 30 mL/kg of excess body fat because of ventricular dilation and increases in stroke volume. The resulting increased left ventricular wall stress leads to hypertrophy, reduced compliance, and impairment of left ventricular filling (diastolic dysfunction) with elevated left ventricular diastolic pressure, and pulmonary edema.35 When left ventricular wall thickening fails to keep pace with dilation, systolic dysfunction (“obesity cardiomyopathy”) and eventual biventricular failure results (Fig. 44-2).
Obesity accelerates atherosclerosis. Symptoms such as angina or exertional dyspnea occur only occasionally because morbidly obese patients often have very limited mobility and may appear asymptomatic even when they have significant cardiovascular disease.
Obesity accelerates atherosclerosis. Symptoms such as angina or exertional dyspnea occur only occasionally because morbidly obese patients often have very limited mobility and may appear asymptomatic even when they have significant cardiovascular disease.
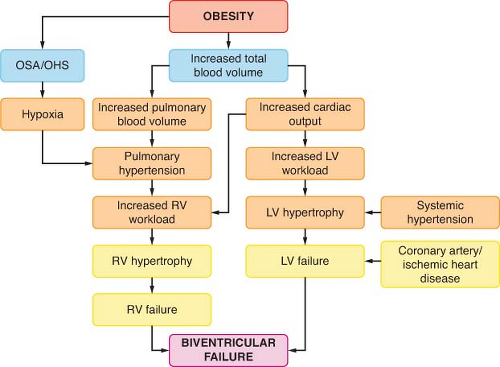 Figure 44.2. Interrelationship of cardiovascular and pulmonary sequelae of obesity. OSA, obstructive sleep apnea; OHS, obesity hypoventilation syndrome; LV, left ventricular; RV, right ventricular. |
Blood flow to fat is 2 to 3 mL/100 g of tissue. An excess of fat requires an increase in cardiac output, to parallel an increase in oxygen consumption. This leads to a systemic arteriovenous oxygen difference that remains normal or slightly above normal. Intraoperative ventricular failure may occur from rapid intravenous fluid administration (indicating left ventricular diastolic dysfunction), the negative inotropism of anesthetic agents, or pulmonary hypertension precipitated by hypoxia or hypercapnia. Cardiac dysrhythmias may be precipitated by fatty infiltration of the conduction system, hypoxia, hypercapnia, electrolyte imbalance, coronary artery disease, increased circulating catecholamines, OSA, and myocardial hypertrophy. Frequent ECG findings seen in morbidly obese patients include low QRS voltage, multiple criteria for left ventricular hypertrophy (LVH) and left atrial enlargement, and T-wave flattening in the inferior and lateral leads.36 In addition, there is a leftward shift of the P-wave, QRS complex, and T-wave axes, lengthening of the corrected QT interval, and prolongation of the QT interval. Substantial weight reduction reverses many of these ECG abnormalities.37
Cardiac output rises faster in response to exercise in the morbidly obese and is often associated with a rise in left ventricular end-diastolic pressure and pulmonary capillary wedge pressure. Similar changes occur during the perioperative period, which should prompt a low threshold for performing detailed cardiac investigations. Many obese patients have mild-to-moderate hypertension, with a 3 to 4 mm Hg increase in systolic and a 2 mm Hg increase in diastolic arterial pressure for every 10 kg of weight gained. Normotensive obese patients have reduced systemic vascular resistance, which rises with the onset of hypertension. Their expanded blood volume causes an increased cardiac output with a lower calculated systemic vascular resistance for the same level of arterial blood pressure. The renin–angiotensin system plays a major role in the hypertension of obesity by increased circulating levels of angiotensinogen, aldosterone, and angiotensin-converting enzyme. As little as 5% reduction in body weight leads to a significant reduction in renin–angiotensin activity in both plasma and adipose tissue, contributing to a reduction in blood pressure.38
Obese patients have a normal-to-increased level of sympathetic nervous system activity, which predisposes to insulin resistance, dyslipidemia, and hypertension.35,39 These obesity-induced comorbidities are responsible for the increased cardiovascular risk in obese patients.40,41 Insulin resistance enhances the pressor activity of norepinephrine and angiotensin II. Hyperinsulinemia further activates the sympathetic nervous system, causing sodium retention and contributes to obesity-induced hypertension. Hypertension causes concentric hypertrophy of the ventricle in normal-weight individuals but causes eccentric dilation in obese individuals. It is associated with increased preload and stroke work. The combination of obesity and hypertension causes left ventricular wall thickening and a larger heart volume; therefore, there is increased likelihood of cardiac failure (Fig. 44-3).
Obese individuals are also prone to cardiovascular disease because adipose tissue releases a large number of bioactive mediators. These can result in abnormal lipids, insulin resistance, inflammation, and coagulopathies.40,41 Obese individuals have higher levels of fibrinogen (a marker for the inflammatory process of atherosclerosis), factor VII, factor VIII, von Willebrand factor, and plasminogen activator inhibitor-1 (PAI-1). Increased levels of fibrinogen, factor VII, factor VIII, and hypofibrinolysis are associated with hypercoagulability. High factor VIII levels are associated with increased cardiovascular mortality. Increased fasting triglyceride levels correlate with increased factor VII concentrations, and postprandial lipemia causes activation of factor VII. Endothelial dysfunction induced by insulin increases von Willebrand factor and factor VIII levels, predisposing to fibrin formation. Increased secretion of PAI-1 inhibits the fibrinolytic system and is associated with visceral obesity.42
Gastrointestinal System
Gastric volume and acidity are increased, hepatic function is altered, and drug metabolism is adversely affected by obesity. Many fasting morbidly obese patients who present for elective surgery have gastric volumes in excess of 25 mL and gastric fluid pH <2.5 (the generally accepted volume and pH indicative of high risk for pneumonitis should regurgitation and aspiration occur). Delayed gastric emptying occurs because of increased abdominal mass that causes antral distension, gastrin release, and a decrease in pH with parietal cell secretion.43,44 Abdominal obesity increases intragastric pressure, increasing the frequency of transient lower esophageal sphincter relaxation, and/or hiatal hernia formation. An increase of >3.5 kg/m2 in BMI is associated with a 2.7-fold increase in risk for developing new reflux symptoms.43 An increased incidence of hiatal hernia and gastroesophageal reflux further increase aspiration risk.
Gastric emptying is faster with high-energy content intake such as fat emulsions, but because of larger gastric volume (up to 75% larger), the residual volume is increased. The combination of hiatal hernia, gastroesophageal reflux, and delayed gastric emptying, coupled with increased intra-abdominal pressure and high volume/low pH gastric content, puts the obese patient at risk for an increased incidence of severe pneumonitis should aspiration occur. Unpremedicated, nondiabetic fasting obese surgical patients who are free from significant gastroesophageal pathology are unlikely to have high volume, low pH gastric contents after routine preoperative fasting.45 They should follow the same fasting guidelines as nonobese patients and be allowed to drink clear liquids up until 2 hours before elective surgery.46 Weight loss significantly improves gastroesophageal reflux symptoms.47
Peculiar morphologic and biochemical abnormalities of the liver are associated with obesity and include fatty infiltration (high prevalence of nonalcoholic fatty liver disease or NAFLD), inflammation (nonalcoholic steatohepatitis or NASH), focal necrosis, and cirrhosis. Fatty infiltration reflects the duration rather than the degree of obesity. Histologic and liver function test abnormalities are relatively common, but clearance usually is not reduced. Abnormal liver function tests are seen in up to one-third of obese patients who have no evidence of concomitant liver disease. The most common abnormality is an increased ALT. Despite these histologic and enzymatic changes, no clear correlation exists between liver function abnormalities and the capacity of the liver to metabolize drugs.48 Morbidly obese patients who have undergone intestinal bypass surgery have a particularly high prevalence of hepatic dysfunction and cholelithiasis. This is also common in the general obese population due to abnormal cholesterol metabolism. The high prevalence of NAFLD, NASH, and cirrhosis necessitates careful assessment for pre-existing liver disease in obese patients scheduled for surgery. Features suggestive of NASH include hepatomegaly, elevated liver enzymes, and abnormal liver histology (steatosis, steatohepatitis, fibrosis, and cirrhosis).40
Renal and Endocrine Systems
Impaired glucose tolerance in the morbidly obese is reflected by a high prevalence of type II diabetes mellitus as a result of resistance of peripheral adipose tissue to insulin.35 Many obese patients have an abnormal glucose tolerance test, and the relative risk of developing diabetes increases by 25% for every 1 kg/m2 increase in BMI above 22 kg/m2.35 Hyperglycemia, insulin resistance, and diabetes predispose obese patients to wound infections and an increased risk of myocardial infarction. Exogenous insulin may be required perioperatively even in obese patients with type II diabetes mellitus to oppose the catabolic response to surgery. In addition to these concerns, subclinical hypothyroidism occurs in about 25% of all morbidly obese patients. Thyroid-stimulating hormone levels are frequently elevated, suggesting the possibility that obesity leads to a state of thyroid hormone resistance in peripheral tissues. Hypothyroidism may be associated with hypoglycemia, hyponatremia, and impaired hepatic drug metabolism.
Obesity is associated with glomerular hyperfiltration as evidenced by increased renal blood flow and an increased glomerular filtration rate. Excessive weight gain increases renal tubular reabsorption and impairs natriuresis through activation of the sympathetic and renin–angiotensin systems as well as physical compression of the kidney. With prolonged obesity, there may be a loss of nephron function, with further impairment of natriuresis and further increases in arterial pressure. Obesity-related glomerular hyperfiltration decreases after weight loss, which decreases the incidence of overt glomerulopathy.49
Metabolic Syndrome
The metabolic syndrome, sometimes referred to as syndrome X and insulin resistance syndrome, is a cluster of metabolic abnormalities associated with an increased risk of diabetes and cardiovascular events. Individuals with this syndrome have up to a fivefold greater risk of developing type 2 diabetes mellitus (if not already present) and are also twice as likely to die from a myocardial infarction or stroke compared with those without the syndrome.50 There are several diagnostic guidelines for metabolic syndrome.51 The most widely used is the one delineated by the revised 2004 National Cholesterol Education Program and the American Heart Association (NCEP II/AHA),52 which defines metabolic syndrome when three out of the following five conditions exist: (1) central obesity: Waist circumference ≥102 cm (≥40 in) in males, ≥88 cm (≥35 in) in females; (2) dyslipidemia: Triglycerides ≥150 mg/dL; (3) dyslipidemia: HDL ≤40 mg/dL in males, ≤50 mg/dL in females; (4) hypertension: ≥130/85 mm Hg
or use of antihypertensives; (5) elevated fasting glucose: ≥100 mg/dL (≥5.6 mmol/L) or use of medication for hyperglycemia. Weight loss and lifestyle changes, such as following a Mediterranean diet with high intake of fruits, vegetables, and fiber, improve the metabolic syndrome features.50,53
or use of antihypertensives; (5) elevated fasting glucose: ≥100 mg/dL (≥5.6 mmol/L) or use of medication for hyperglycemia. Weight loss and lifestyle changes, such as following a Mediterranean diet with high intake of fruits, vegetables, and fiber, improve the metabolic syndrome features.50,53
Pharmacology
Pharmacologic Principles
General pharmacokinetic principles dictate, with certain exceptions, that drug dosing should take into consideration the volume of distribution (VD) for administration of the loading dose, and the clearance for the maintenance dose.54 A drug that is mainly distributed to lean tissues should have the loading dose calculated on the basis of LBW. If the drug is equally distributed between adipose and lean tissues, dosing should be calculated on the basis of TBW. For maintenance, a drug with similar clearance values in both obese and nonobese individuals should have the maintenance dose calculated on the basis of LBW. However, a drug whose clearance increases with obesity should have the maintenance dose calculated according to TBW.
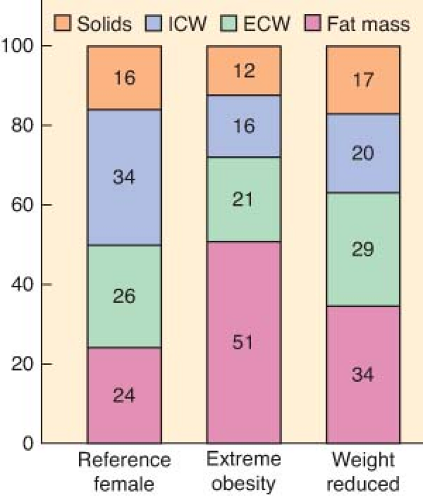
The relative volume of the central compartment in which drugs are first distributed remains unchanged in obese patients, but absolute body water content is decreased. Lean body and adipose tissue mass are increased, affecting lipophilic and polar drug distribution (Fig. 44-4). The VD in obese patients is affected by multiple factors including reduced total body water, increased total body fat, increased lean body mass, altered protein binding, increased blood volume, increased cardiac output, increased blood concentrations of free fatty acids, triglycerides, cholesterol, and α1-acid glycoprotein, lipophilicity of the drug, and organomegaly.6 Increased redistribution of a drug prolongs its elimination half-life even when clearance is unchanged or increased. Hyperlipidemia and an increased concentration of α1-acid glycoprotein may affect protein binding, leading to a reduction in free drug concentration. Plasma albumin and total plasma protein concentrations and binding are not significantly changed by obesity, but when compared with normal-weight individuals, a relative increase in plasma protein binding may be evident. Splanchnic blood flow, blood volume, and cardiac output are all increased in obese patients. In contrast to the expected decrease in bioavailability of orally administered medications because of increased splanchnic blood flow, there is no significant difference in absorption and bioavailability when comparing obese and normal-weight subjects. Drugs that undergo phase I metabolism (oxidation, reduction, hydrolysis) are generally unaffected by changes induced by obesity, whereas phase II reactions (glucuronidation, sulfation) are enhanced.6
Histologic abnormalities of the liver are common in the obese, with concomitant deranged liver function tests, but drug clearance is not usually affected. Renal clearance of drugs is increased in obesity because of increased renal blood flow and glomerular filtration rate.49,55 As a result of the increases in glomerular filtration rate and tubular secretion, drugs such as cimetidine and aminoglycoside antibiotics that depend on renal excretion may require increased dosing. Highly lipophilic substances such as barbiturates and benzodiazepines show significant increases in VD for obese individuals.6 These drugs have a more selective distribution to fat stores and therefore a longer elimination half-life but with comparable clearance values to normal individuals. Less lipophilic compounds have little or no change in VD with obesity. Exceptions to this rule include the highly lipophilic drugs digoxin, procainamide, and remifentanil.56,57,58 Drugs with weak or moderate lipophilicity may be dosed on the basis of LBW. Adding 20% to the estimated IBW dose of hydrophilic medications is sufficient to include the obese patient’s extra lean mass. Nondepolarizing muscle relaxants can be dosed in this manner.
Increased blood volume in the obese patient decreases plasma concentrations of rapidly injected intravenous drugs. Fat, however, has poor blood flow, and doses calculated on actual body weight could lead to excessive plasma concentrations. Calculating initial doses based on LBW with subsequent doses determined by pharmacologic response to the initial dose is a reasonable approach. Repeated injections may accumulate in fat, leading to a prolonged response because of subsequent release from this large depot.
Specific Drugs
Patients’ usual medications should be continued until the time of surgery, with the possible exception of certain antihypertensives, insulin, and oral hypoglycemics. Antibiotic prophylaxis is usually indicated because of an increased incidence of wound infections in the obese.59 Anxiolysis and prophylaxis against both aspiration pneumonitis and deep vein thrombosis (DVT) should be addressed preoperatively. Oral benzodiazepines are reliable for anxiolysis and sedation. Intravenous midazolam can also be titrated in small doses for anxiolysis during the immediate preoperative period. Dexmedetomidine, because of its minimal respiratory depressant effects, should be considered. Pharmacologic intervention with H2-receptor antagonists, nonparticulate antacids, or proton pump inhibitors will reduce gastric volume, acidity, or both, thereby reducing the risk and severity of aspiration pneumonitis. Guidelines for dosing of common intravenous drugs utilized during anesthesia are presented in Table 44-3.
Table 44-3. Intravenous Drug Dosing in Obesity | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Preoperative Evaluation
Airway
Preoperative airway assessment in obese patients is of paramount importance. In an analysis of closed malpractice claims in the United States related to airway management, obesity played a significant role in a large number of cases.70 Anatomic changes associated with obesity that contribute to a potentially difficult airway include limited movement of the atlantoaxial joint and cervical spine by upper thoracic and low cervical fat pads; excessive tissue folds in the mouth and the pharynx; a short, thick neck; a thick submental fat pad; suprasternal, presternal, and posterior cervical fat; and large breasts in females. Excess pharyngeal tissue deposited in the lateral pharyngeal walls may not be noticed during routine airway examination. The history obtained from the patient and examination of previous records may help predict airway difficulties.
Cardiopulmonary Systems
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree