Fig. 1
Opioids were applied in the volar forearm of volunteers by dermal microdialysis. Intensity of opioid-induced maximum itch is shown in (a) (visual analog scale 0–10, mean ± SEM). (b) Peak mast cell tryptase release during stimulation with the opioids is shown (mean ± SEM). Only low affinity opioids meperidine (40.4 mM) and morphine (3.11 mM) caused tryptase release from mast cells. The potent opioids alfentanil (1.2 mM), sufentanil (0.12 mM), and remifentanil (2.65 mM) provoked neither itch nor tryptase release (modified from Blunk et al. 2004)
Central inhibition of itch can also be achieved by cold stimulation (Bromm et al. 1995). In addition, cooling has a peripheral inhibitory effect: histamine-induced activation of nociceptors can be reduced by cooling (Mizumura and Koda 1999). Also in humans, cooling of a histamine-treated skin site reduced the activity of the primary afferents and decreased the area of “itchy skin” or “hyperknesis” around the application site (Heyer et al. 1995). Unexpectedly, there is an initial increase of itch intensity upon cooling the histamine application site (Pfab et al. 2006) that can be used as experimental model for central imaging (Napadow et al. 2014). Conversely, tonic warming the skin would lead to an exacerbation of itch. However, as soon as the heating becomes painful, central inhibition of pruritus will counteract this effect (Schmelz 2002).
Recent work on antipruritic effects of subpopulations of primary nociceptive afferents indicates that especially the input from the VGLUT2-positive subpopulation is crucial to explain inhibition of itch behavior by painful stimuli (Lagerstrom et al. 2010; Liu et al. 2010). When VGLUT2 and, thereby, glutamate release in NaV1.8-positive nociceptors was deficient, inflammatory and neuropathic pain responses were grossly abolished, but spontaneous scratching behavior and increased experimental itch were massively enhanced (Liu et al. 2010). Most interestingly, capsaicin-induced pain behavior was changed into scratching behavior in these mice suggesting that the lack of noxious input via VGLUT2-positive nociceptors disinhibited itch (Liu et al. 2010). The exact nature of the crucial nociceptor class is still unclear, as another group did not find increased scratching when VGLUT2 was knocked out in NaV1.8-positive but in TRPV1-positive primary afferent neurons (Lagerstrom et al. 2010). It will be of major interest to further characterize the nociceptor class having a crucial itch-inhibiting spinal effect.
It is important to note that we have so far followed the ideas of two separate populations for itch and pain. This segregation is actually required for the genetic approaches described above. Unfortunately, several aspects of itch generation such as pruritus induced by activation of polymodal nociceptors or spatial stimulus characteristics switching pain to itch cannot be sufficiently explained by this approach.
1.2 Intensity and Pattern Theory of Itch
Numerous neuronal markers that were linked to itch, but not pain processing, support the specificity theory of itch. However, as indicated above, there is evidence that itch can also be induced by activation of nociceptors. Nociceptors could provoke itch either by an intensity coding (“intensity theory”) or by a particular population encoding (“pattern theory”) (Namer and Reeh 2013; Handwerker 2014). Albeit this general question might appear purely academic, it is crucial to provide the most promising research approach to identify pharmacological targets in chronic itch and pain.
1.2.1 Non-histaminergic Itch
Histamine application causes a local wheal response surrounded by an area of vasodilation (“axon reflex erythema”) (Lewis et al. 1927). This vasodilation is induced by neuropeptide release from mechano-insensitive C-fibers (Schmelz et al. 2000; Geppetti and Holzer 1996). The absence of an axon reflex flare therefore suggests that the itch is independent of histamine-sensitive C-fibers. Indeed, itch was induced by papain in an early study in the absence of a flare response indicating a histamine-independent action (Hägermark 1973). Itch without axon reflex flare can also be elicited by weak electrical stimulation (Shelley and Arthur 1957; Ikoma et al. 2005), providing further evidence that the sensation of itch can be dissociated from cutaneous vasodilation.
Cowhage spicules inserted into human skin produce itch in an intensity which is comparable to that following histamine application (LaMotte et al. 2009; Sikand et al. 2009), but is not accompanied by an axon reflex erythema and unresponsive to histamine (H1) blocker (Johanek et al. 2007). The active compound, the cysteine protease muconain, has been identified lately and has shown to activate proteinase-activated receptor 2 (PAR 2) and even more potently PAR 4 (Reddy et al. 2008). Interestingly, mechano-responsive “polymodal” C-fiber afferents, the most common type of afferent C-nociceptors in human skin (Schmidt et al. 1995), can be activated by cowhage in the cat (Tuckett and Wei 1987), in nonhuman primates (Johanek et al. 2007, 2008), and in human volunteers (Namer et al. 2008) (Fig. 2).
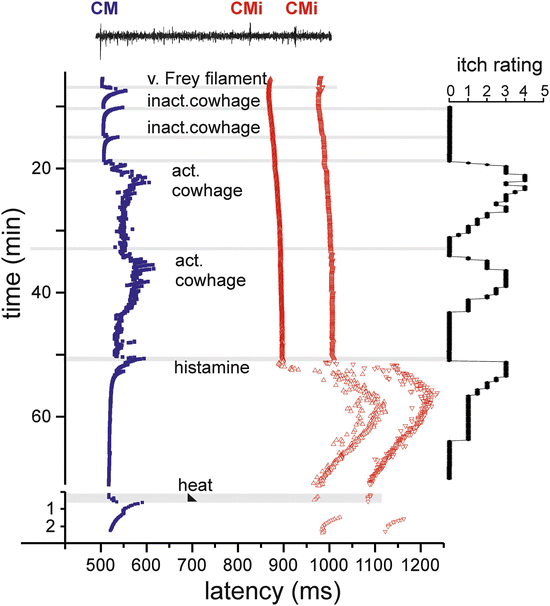

Fig. 2
Specimen of a multifiber recording from a mechano-responsive (CM, blue) and two mechano-insensitive nociceptors (CMi, red) in human (raw signal with marked action potential on top). Conduction latencies of these three marked fibers (filled square, open triangles) in response to successive electrical stimulation at the receptive field are plotted from top to bottom. When activated by mechanical (v. Frey filament, inactivated cowhage spicules), chemical (active cowhage, histamine), or heat test stimuli (black triangle), C-fibers exhibit activity-dependent increase of response latency followed by a gradual normalization (“marking”). The mechano-responsive fiber is activated during mechanical stimulation with the v. Frey filament and during application of inactive cowhage, but lasting activation is only seen after application of active cowhage. In contrast, the mechano-insensitive fibers do not respond to cowhage stimulation, but are activated following histamine iontophoresis. At the right side of the panel, the itch ratings of the subject are depicted which were assessed during this experiment. Ratings are given on a numerical rating scale from 0 (0 = no itch) to 10 (10 = maximal imaginable itch). Inactive cowhage does not evoke any itch, whereas active cowhage and histamine evoke itch similarly in time course and maximum mirroring nicely the activation pattern of the fibers. Modified from Namer et al. (2008)
Given that cowhage spicules can activate a large proportion of polymodal nociceptors, we face a major problem to explain why activation of these fibers by heat or by scratching actually inhibits itch, whereas activation by cowhage produces it. On the other hand, data from monkey suggest that mechano-heat-sensitive C-nociceptors with a fast response to heating (“QC”) might play a more important role in mediating cowhage-induced itch (Johanek et al. 2008). One might therefore still hypothesize that there is a certain selectivity among mechano-sensitive C-nociceptors for cowhage that would allow the central nervous system to separate nociceptive from pruriceptive stimuli (LaMotte et al. 2014). Along the same lines, in particular QC-nociceptors, but not mechano-insensitive nociceptors, were activated by beta-alanine (Wooten et al. 2014), the activator of MrgprD that provokes itch in humans.
1.2.2 Encoding Itch by Patterns of Activated Nociceptors
Considering nociceptors being involved in generating itch, a population code has been postulated (“pattern theory”) (Handwerker 2014; Akiyama and Carstens 2013; McMahon and Koltzenburg 1992) in which only a subpopulation of nociceptors can also be activated by pruritic stimuli, whereas pure nociceptors are only responsive to algogens. Accordingly, itch will be felt when only the first subpopulation is responding, but pain when both populations are active.
The encoding of itch by nociceptors has also been proposed to be based on a spatial code (Schmelz and Handwerker 2013) based on the itch induced by capsaicin being applied very localized on a cowhage spicule into the epidermis (Sikand et al. 2009). The highly localized stimulation in the epidermis strongly activates some of the local nociceptors, while their immediate neighbors remain silent resulting in a mismatch signal of activation and absence of activation from this site. It has thus been hypothesized that this mismatch might be perceived by the central nervous system as itch (Schmelz and Handwerker 2013; Namer et al. 2008). Interestingly, this result also speaks against capsaicin’s pain specificity (Ross 2011). Teleologically, it is obvious that scratching behavior in the case of a highly localized superficial noxious focus is an adequate response as it can eliminate the presumed cause. Moreover, scratching activates all the mechano-sensitive nociceptors in the stimulated area, and thus, the mismatch signal of activated and nonactivated nociceptors at this site is terminated. Therefore, it needs to be pointed out that pruritus cannot only be explained by itch-specific or itch-selective neurons (LaMotte et al. 2014) along the specificity theory. In addition, the pure spatial pattern of activated nociceptors might similarly underlie the itch sensation without any requirement of itch-specific primary afferent neurons.
2 Central and Peripheral Sensitization in Itch and Pain
Spontaneous itch and pain are of paramount clinical relevance as they correlate to the main complaint of chronic itch and pain patients. It is highly interesting that the patterns of peripheral and central sensitization linked to chronic pain and itch are remarkably similar.
2.1 Central Sensitization
Activity in chemo-nociceptors leads not only to acute pain but, in addition, can sensitize second-order neurons in the dorsal horn, thereby leading to increased sensitivity to pain (hyperalgesia). Two types of mechanical hyperalgesia can be differentiated. Normally painless touch sensations in the uninjured surroundings of a trauma are felt as painful “touch- or brush-evoked hyperalgesia” or allodynia. Though this sensation is mediated by myelinated mechanoreceptor units, it requires ongoing activity of primary afferent C-nociceptors (Torebjörk et al. 1996). The second type of mechanical hyperalgesia results in slightly painful pinprick stimulation being perceived as being more painful in the secondary zone around a focus of inflammation. This type has been called “punctate hyperalgesia” and does not require ongoing activity of primary nociceptors for its maintenance. It can persist for hours following a trauma, usually much longer than touch- or brush-evoked hyperalgesia (LaMotte et al. 1991).
In itch processing, similar phenomena have been described: touch- or brush-evoked pruritus around an itching site has been termed “itchy skin” (Bickford 1938; Simone et al. 1991). Like allodynia, it requires ongoing activity in primary afferents and is most probably elicited by low-threshold mechanoreceptors (Aδ-fibers) (Simone et al. 1991; Heyer et al. 1995). Also, more intense prick-induced itch sensations in the surroundings, “hyperknesis,” have been reported following histamine iontophoresis in healthy volunteers (Atanassoff et al. 1999) (Fig. 3).
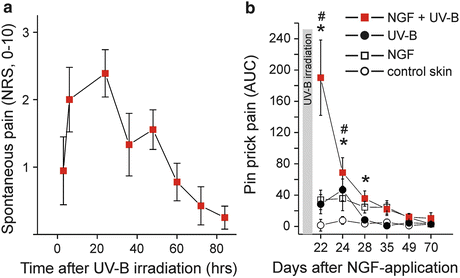

Fig. 3
(a) Pre-sensitization with nerve growth factor (NGF, 1 μg) injected 3 weeks before UV-B irradiation (threefold minimum erythema dose) provoked spontaneous pain ratings following the intensity of the UV-induced inflammation. (b) Hyperalgesia to pinprick stimuli develops following intradermal NGF injection and also for about 3 days after UV-B irradiation. Combined sensitization with NGF and UV-B irradiation causes a supra-additive increase of mechanical hyperalgesia. Modified from Rukwied et al. (2013b)
The existence of central sensitization for itch can greatly improve our understanding of clinical itch. Under the conditions of central sensitization leading to punctuate hyperknesis, normally painful stimuli are perceived as itching. This phenomenon has already been described in patients suffering from atopic dermatitis, who perceive normally painful electrical stimuli as itching when applied inside their lesional skin (Nilsson et al. 2004; Nilsson and Schouenborg 1999). Furthermore, acetylcholine provokes itch instead of pain in patients with atopic dermatitis (Vogelsang et al. 1995), indicating that pain-induced inhibition of itch might be compromised in these patients.
The exact mechanisms and roles of central sensitization for itch in specific, clinical conditions have still to be explored, whereas a major role of central sensitization in patients with chronic pain is generally accepted. It should be noted that in addition to the parallels between experimentally induced secondary sensitization phenomena, there is also emerging evidence for corresponding phenomena in patients with chronic pain and chronic itch. In patients with neuropathic pain, it has been reported that histamine iontophoresis resulted in burning pain instead of pure itch which would be induced by this procedure in healthy volunteers (Birklein et al. 1997; Baron et al. 2001). This phenomenon is of special interest as it demonstrates spinal hypersensitivity to C-fiber input. Conversely, normally painful electrical, chemical, mechanical, and thermal stimulation is perceived as itching when applied in or close to lesional skin of atopic dermatitis patients (Heyer et al. 1995; Steinhoff et al. 2003).
Ongoing activity of pruriceptors, which might underlie the development of central sensitization for itch, has already been confirmed microneurographically in a patient with chronic pruritus (Schmelz et al. 2003a). Thus, there is emerging evidence, for a role of central sensitization for itch in chronic pruritus.
While there is obviously an antagonistic interaction between pain and itch under normal conditions, the patterns of spinal sensitization phenomena are surprisingly similar. It remains to be established whether this similarity will also include the underlying mechanism which would also implicate similar therapeutic approaches such as gabapentin (Dhand and Aminoff 2014) or clonidine (Elkersh et al. 2003) for the treatment of neuropathic itch.
2.2 Peripheral Sensitization
There is cumulative evidence for a prominent role of nerve growth factor (NGF)-induced sensitization of primary afferents in both chronic itch and pain: increased levels of NGF were found in chronic itch patients suffering from atopic dermatitis or psoriasis (Toyoda et al. 2002, 2003; Tominaga et al. 2009; Yamaguchi et al. 2009). Similarly, there is clear evidence for a major role of NGF in chronic inflammatory pain (Chevalier et al. 2013; Watanabe et al. 2011; Barcena de Arellano et al. 2011). Moreover, blocking NGF by specific antibodies proved to be analgesic in the chronic pain patients (Lane et al. 2010; Sanga et al. 2013). Anti-NGF strategies also were successful in animal models of chronic itch (Tominaga and Takamori 2014). It is therefore not surprising that intradermally injected NGF not only causes hyperalgesia to heat and mechanical stimuli in volunteers (Hirth et al. 2013; Rukwied et al. 2010) but also sensitizes for cowhage-induced itch (Rukwied et al. 2013c). Intracutaneous NGF injection does not induce visual inflammatory responses in human (Rukwied et al. 2010), but interestingly, when combined with an inflammatory pain model (UV-B sunburn), the subjects report of spontaneous pain (Fig. 3) and pronounced hyperalgesia (Rukwied et al. 2013b) that also includes axonal hyperexcitability (Rukwied et al. 2013a). These results nice match the analgesic effects of anti-NGF in chronic inflammatory pain that are not accompanied by reduced signs of inflammation (Lane et al. 2010). Therefore, it emerges that neurotrophic factors such as NGF can change expression patterns of primary afferent nociceptors such that their ability to signal pain or itch by local inflammatory mediators is increased. This increase might be based on higher discharge frequencies linked to sensitized transduction, but also to axonal hyperexcitability.
3 Perspectives: Mechanisms for Itch or Pain in Neuropathy and Chronic Inflammation
Finally, the current concepts differentiating itch and pain need to be evaluated in view of the obvious clinical questions concerning the development of itch or pain after neuropathy or in chronic inflammatory diseases. It is remarkable that some neuropathic conditions such as postherpetic neuralgia and diabetic neuropathy are primarily linked to pain symptoms whereas patients suffering from notalgia paresthetica or brachioradial pruritus mainly report chronic itch (Table 1).
Table 1
Summary of neuropathic conditions and their dominant symptoms
Pain | Itch | |
|---|---|---|
Postherpetic neuralgia | +++ | ++ |
Diabetic neuropathy | ++(+) | + |
Meralgia paresthetica | +++ | (+) |
Notalgia paresthetica | (+) | +++ |
Brachioradial pruritus | (+) | +++ |
It is important to note that more than 25 % of patients with neuropathic pain conditions such as postherpetic neuropathy also report itch (Oaklander et al. 2003). According to the specificity or selectivity theory, one would hypothesize that the mediators being released in diabetic neuropathy or postherpetic neuralgia determine to which extent itch-selective or itch-specific primary afferents are excited. Moreover, itching neuropathic conditions such as nostalgia paresthetica and brachioradial pruritus should be differentiated from painful meralgia paresthetica by primary activation of pruriceptors rather than nociceptors. However, it is completely unclear how such differentiation could be mediated for very similar peripheral neuropathic conditions. Possibly, specific pruriceptors only play a minor role under these conditions. In contrast, the spatial pattern of nociceptor activation might provide the crucial input: if only few scattered axons are spontaneously active, their input might mimic the one of scattered nociceptors being activated by cowhage spicules in the epidermis, whereas activation of numerous nociceptors of a peripheral nerve would result in pain. Thus, such itch sensation would be generated by the particular spatial code of activated nociceptors (Schmelz and Handwerker 2013; Namer et al. 2008). Accordingly, scattered activation of epidermal nociceptors might also occur in some chronic inflammatory diseases such as atopic dermatitis and explain the difference between itching and painful symptoms. If this hypothesis would be correct, the treatment of neuropathic itch and pain would have essentially identical therapeutic targets and mechanisms rather than itch or pain specific. Thus, the implications of theoretical concepts of itch are, unexpectedly, of major clinical relevance. It will therefore be of major interest for both clinicians and basic researchers to determine which fiber class generates the peripheral input for chronic itch conditions.
References
Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, Wessberg J (2014) Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci 34(8):2879–2883. doi:10.1523/JNEUROSCI. 2847-13.2014 CrossRefPubMedCentralPubMed
Akiyama T, Carstens E (2013) Neural processing of itch. Neuroscience 250:697–714. doi:10.1016/j.neuroscience.2013.07.035 CrossRefPubMedCentralPubMed
Andrew D, Craig AD (2001) Spinothalamic lamina 1 neurons selectively sensitive to histamine: a central neural pathway for itch. Nat Neurosci 4:72–77CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






