Fig. 27.1
Anterior view of the liver. IVC inferior vena cava, PHA proper hepatic artery, MPV main portal vein, CBD common bile duct
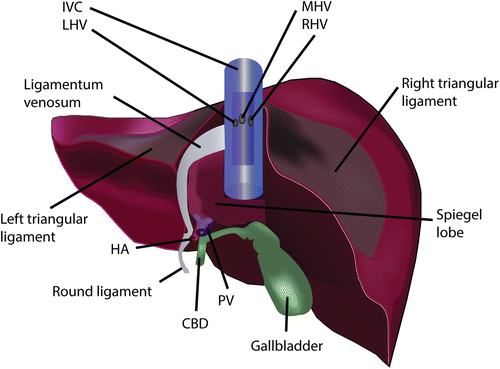
Fig. 27.2
Posterior view of the liver. The ligamentum venosum ends near the left hepatic vein (LHV) and is an important landmark for finding the LHV. IVC inferior vena cava, MHV middle hepatic vein, RHV right hepatic vein, HA hepatic artery, PV portal vein, CBD common bile duct
The ligamentum venosum is connected with the lesser gastric curvature by the lesser omentum in which we sometimes find an accessory left hepatic artery accompanied by the presence of the vagus nerve branch. More caudally the lesser omentum is bordered by the hepatoduodenal ligament. In the hepatoduodenal ligament we encounter the common bile duct at the most right sided border. Proximally towards the liver the common bile duct becomes the common hepatic duct after the insertion of the cystic duct. The common hepatic artery branches off at the left inferior border above the duodenum into a right and left hepatic artery. A middle hepatic artery can sometimes be seen branching off the right hepatic artery before the right hepatic artery dives behind the common hepatic duct. In rare circumstances the right hepatic artery is found anterior to the common hepatic duct. Aberrant arterial variations can occur and are known as accessory when they complement a normal arterial anatomy or as replaced when they replace the normal anatomy. For instance an accessory left hepatic artery from the left gastric artery can be seen in the lesser omentum and this is in coexistence with a normal left hepatic artery. Similarly a replaced right hepatic artery coming from the superior mesenteric artery can be found running behind and parallel to the portal vein on the right side of the hepatoduodenal ligament. The portal vein is usually the most dorsal structure found behind the bile duct and arterial structures. The portal vein is formed by the confluence of the superior mesenteric vein and the splenic vein. The portal vein along with the artery and the bile duct forms the portal triad. The whole triad is surrounded by a connective tissue known as the glissonean sheath. At the hilum this is also known as the hilar plate. Externally this sheath encapsulates the liver. Cranially it forms the two triangular ligaments attaching it to the diaphragm which in turn form the coronary ligament towards the center. It is only at the bare area between the right and left coronary ligament where the liver is not encapsulated. Underneath the bare area towards the IVC is where the main hepatic veins are found.
Posteriorly the liver wraps around the inferior vena cava (IVC). This is incomplete in most cases with on the left side a protruding lobe known as the Spiegel lobe (Fig. 27.2) and on the right anterior side the paracaval portion. Together the Spiegel and the paracaval portion are known as the caudate lobe. The two portions are connected on the right side of the vena cava by a hepatocaval ligament or in lesser extent by liver parenchyma if the paracaval portion wraps completely around the right side. It is by dividing this ligament during hepatectomy or during right lobe graft procurement that the right hepatic vein becomes exposed. With the exception of the caudate lobe the liver venous drainage into the IVC is by the three hepatic veins at the suprahepatic anterior border: the right hepatic vein (RHV), middle hepatic vein (MHV), and the left hepatic vein (LHV). The caudate lobe drains directly into the IVC by retrohepatic accessory hepatic veins which are usually small. These veins are divided in IVC sparing transplant hepatectomy. Sometimes these caudate lobe branches cover a large enough drainage area in living donors that they need to be reattached in the implantation process in which case they will be labeled as right inferior or accessory hepatic veins.
In 1957 Couinaud published his work on liver anatomy and as of today his work still stands as the basis for functional liver anatomy [5, 6]. He divided the liver into eight segments using the portal and hepatic vein branches. The liver is first divided into two hemi livers by the portal bifurcation. This division can be found in an imaginary line formed from the gallbladder fossa towards the space between the RHV and MHV. This line is known as Cantie’s line. It is an important landmark for right lobe hepatectomy as it runs anteriorly towards the MHV. The right liver is then further divided into a right anterior sector supplied by the right anterior portal branch (secondary division) and a right posterior sector supplied by the right posterior branch. Both sectors are separated by the right hepatic vein. Tertiary division of the portal veins will separate the sectors in a superior and inferior part i.e. the right posterior sector is segment VII superiorly and segment VI inferiorly and segment VIII with segment V respectively for the right anterior branch. For the left hemiliver the secondary portal division divides the liver into a medial and a lateral sector. The medial sector being segment IV and the lateral sector being the left lateral lobe consisting of segment II and III. Tertiary division divides Segments IV A superiorly and IV B inferiorly. The same goes for the lateral sector with segment II superior and segment III inferior. Between the right anterior sector and the left medial sector lies the middle hepatic vein. This is an important landmark for right lobe resection in live donor surgery as mentioned earlier. Thus the middle hepatic vein drains part of segment VIII and V which is often reconstructed in right lobe living donor transplantation without MHV. The aim is to optimize the outflow of the graft and hence prevent a small for size syndrome [7]. The medial sector is divided from the lateral sector by the falciforme ligament and is drained by the left hepatic vein. As mentioned earlier, the caudate lobe or segment 1 lies paracaval and is separated from the rest of the liver by the ligamentum venosum. The segmental numbering follows a clockwise rotation from the left lateral superior position toward right medial superior if looking at the liver from above.
Surgical Procedures
Donor Surgery
The procedure of liver transplant usually starts with the donor procurement. An ideal deceased donor is usually younger than 50 years, has absence of steatosis, is hemodynamically stable, without abdominal trauma and has good renal function with good diuresis. The procedure is usually a multi-organ retrieval with cardiothoracic participation. Donor and recipients are usually matched by ABO types and by size compatibility. Larger grafts can be split for two recipients such as left lateral segment for a pediatric and an extended right lobe for an adult recipient. Two hemi-livers for two adults are also possible but less common due to higher biliary and vascular complications and higher PNF rates [8, 9]. Several reasons have been suggested as cause for these complications and they include the longer cold ischemia time due to the longer procurement time, the smaller artery sizes, and the loss of the segment 4 branch artery during splitting [9].
Deceased Donor Procurement
For the procuring surgeon the most important aspect of donor procurement once the donor organ is deemed suitable for use is to limit and avoid warm ischemia time in cardiac and brain death donors respectively during the procurement. This is achieved by gaining rapid inflow control for cold preservation fluid installation into the donor body as soon as possible. In cardiac death donation the mandatory 5 min wait time after death declaration inevitably adds up to warm ischemia time. Stricter criteria are now being applied for use of organs with prolonged warm ischemia time amongst many centers in an attempt to decrease the incidence of biliary complications.
Routinely the donor procurement is performed through a combined laparotomy and sternotomy. In brain dead donors when the donor is hemodynamically stable, preparatory work prior to cannulation can be done to ensure a fastidious procurement once flushing has started. The liver is first inspected for color, texture, and aberrant arterial anatomy. The left triangular ligament is divided along with the coronary ligament. This maneuver permits early determination for the presence of an accessory left hepatic artery in the lesser omentum. It also allows proximal supra-celiac aortic clamping in case where lungs are also procured. Next the right hemicolon along with the distal small bowel are freed up from their retroperitoneal attachment from right inferolateral towards left medial. This gesture is known as the Cattell Braasch maneuver and together with duodenal mobilization of the second and third portion (Kocher maneuver) permits complete exposure of the distal aorta and inferior vena cava up to the left renal vein. The right superior mesenteric artery (SMA) is in this way also exposed and an aberrant or replaced right hepatic artery if present can be seen coming off the SMA. The distal aorta is encircled for control after division of the inferior mesenteric artery. The inferior mesenteric vein which runs to the left side of the ligament of Treitz can be isolated and cannulated for portal flushing if necessary.
The hepatoduodenal ligament is then dissected with isolation of the common bile duct . The bile duct and gallbladder if present are flushed at the last minute to minimize bilious contamination of other organs such as heart and lungs. This is done by incising in the gallbladder and dividing the CBD with ligation of the distal stump and injecting 100 ml of physiological serum into the gallbladder forcing it to wash out through the CBD. The CBD is also retrograde flushed with 40 ml of physiological solution. This is done to prevent mucosal autolysis of the biliary system. Some centers dissect the hepatic artery down to the splenic artery in the warm. We routinely perform this in the cold or on the back table when the pancreas is separated from the liver. Next the supraceliac aorta is dissected just beneath the right diaphragmatic cruz and encircled ready for proximal clamping. In cases where no lungs are procured the distal thoracic aorta can be dissected after lifting of the left lung and an open clamp can be placed on the distal aorta just beneath the esophagus.
At this point the abdominal team is ready for aortic cannulation, and in agreement with the cardiothoracic team, 100 U/kg of heparin is given. The distal abdominal aorta is cannulated after 3 min of heparin injection and cold flush fluid is infused. We routinely perform 7 L aortic and 3 L portal flushing with HTK solution. In pediatric donors we use UW solution and the volumes are 4 L and 2 L respectively. Simultaneous proximal aortic clamping is performed by the cardiothoracic and abdominal teams. The cardiothoracic team clamps the ascending thoracic aorta and we usually clamp the supra-celiac or distal thoracic aorta depending on whether the lungs are procured. At the same time the flushing preservative fluid is running through the distal abdominal cannula. The right atrium is then opened to vent the effluent preservation fluid. Care must be made as to have a sufficient suprahepatic IVC when incising the right atrium. This is important for piggyback implantation as discussed further. We prefer venting through the right atrium in order to prevent hepatic congestion. At this point anesthesiology hemodynamic support is halted and topical ice is placed over the organs that will be procured. Abdominal procurement is performed after the cardiothoracic organs are removed.
The colon is completely mobilized by dividing the mesocolon from the cecum up to the proximal rectum. The mesentery of the small bowel is divided together with the proximal jejunum at roughly 10 cm from the ligament of Treitz. The bowels are retracted out of the body and placed caudally onto the thighs. This provides good exposure for further procurement. The pancreas is first mobilized together with the spleen from its retroperitoneal attachments. The distal stomach is then divided just before the pylorus. The left gastric artery is cut at the lesser curvature and preserved with the lesser omentum. The aorta is cut just below the SMA with care taken to preserve an adequate cuff for the renal arteries. The left diaphragm is incised and the proximal aorta is then divided at that point. The right diaphragm is then incised and the distal IVC is divided above the left renal vein. The liver and pancreas bloc is then removed from the remaining retroperitoneal attachments. The pancreas is then separated from the liver on the back table by dividing the structures in the hepatoduodenal ligament. Once the kidneys are procured, arterial and venous iliac grafts are then removed. One side of each vascular graft is then packed with the liver.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





