Key Concepts
 The accumulation of morphine metabolites (morphine 3-glucuronide and morphine 6-glucuronide) in patients with kidney failure has been associated with narcosis and ventilatory depression lasting several days.
The accumulation of morphine metabolites (morphine 3-glucuronide and morphine 6-glucuronide) in patients with kidney failure has been associated with narcosis and ventilatory depression lasting several days.
 Rapid administration of larger doses of opioids (particularly fentanyl, sufentanil, remifentanil, and alfentanil) can induce chest wall rigidity severe enough to prevent adequate bag-and-mask ventilation.
Rapid administration of larger doses of opioids (particularly fentanyl, sufentanil, remifentanil, and alfentanil) can induce chest wall rigidity severe enough to prevent adequate bag-and-mask ventilation.
 Prolonged dosing of opioids can produce “opioid-induced hyperalgesia,” in which patients become more sensitive to painful stimuli. Infusion of large doses of (in particular) remifentanil during general anesthesia can produce acute tolerance, in which much larger than usual doses of opioids are required for postoperative analgesia.
Prolonged dosing of opioids can produce “opioid-induced hyperalgesia,” in which patients become more sensitive to painful stimuli. Infusion of large doses of (in particular) remifentanil during general anesthesia can produce acute tolerance, in which much larger than usual doses of opioids are required for postoperative analgesia.
 The neuroendocrine stress response to surgical stimulation is measured in terms of the secretion of specific hormones, including catecholamines, antidiuretic hormone, and cortisol. Large doses of opioids block the release of these hormones in response to surgery more completely than volatile anesthetics.
The neuroendocrine stress response to surgical stimulation is measured in terms of the secretion of specific hormones, including catecholamines, antidiuretic hormone, and cortisol. Large doses of opioids block the release of these hormones in response to surgery more completely than volatile anesthetics.
 Aspirin is unique in that it irreversibly inhibits COX-1 by acetylating a serine residue in the enzyme. The irreversible nature of its inhibition underlies the nearly 1-week duration of its clinical effects (eg, return of platelet aggregation to normal) after drug discontinuation.
Aspirin is unique in that it irreversibly inhibits COX-1 by acetylating a serine residue in the enzyme. The irreversible nature of its inhibition underlies the nearly 1-week duration of its clinical effects (eg, return of platelet aggregation to normal) after drug discontinuation.
Analgesic Agents: Introduction
Regardless of how expertly surgical and anesthetic procedures are performed, appropriate prescription of analgesic drugs, especially opioids and cyclooxygenase (COX) inhibitors, can make the difference between a satisfied and an unsatisfied postoperative patient. Studies have shown that outcomes can be improved when analgesia is provided in a “multimodal” format (typically emphasizing COX inhibitors and local anesthetic techniques while minimizing opioid use) as one part of a well-defined and well-organized plan for postoperative care (see Chapter 48).
Opioids
Opioids bind to specific receptors located throughout the central nervous system and other tissues. Four major opioid receptor types have been identified (Table 10-1): mu (μ, with subtypes μ1 and μ2), kappa (κ), delta (δ), and sigma (σ). All opioid receptors couple to G proteins; binding of an agonist to an opioid receptor causes membrane hyperpolarization. Acute opioid effects are mediated by inhibition of adenylyl cyclase (reductions in intracellular cyclic adenosine monophosphate concentrations) and activation of phospholipase C. Opioids inhibit voltage-gated calcium channels and activate inwardly rectifying potassium channels. Opioid effects vary based on the duration of exposure, and opioid tolerance leads to changes in opioid responses.
| Receptor | Clinical Effect | Agonists |
|---|---|---|
| μ |
|
|
| κ |
|
|
| δ |
|
|
| σ |
|
|
Although opioids provide some degree of sedation and (in many species) can produce general anesthesia when given in large doses, they are principally used to provide analgesia. The properties of specific opioids depend on which receptor is bound (and in the case of spinal and epidural administration of opioids, the location in the neuraxis where the receptor is located) and the binding affinity of the drug. Agonist-antagonists (eg, nalbuphine, nalorphine, butorphanol, and pentazocine) have less efficacy than so-called full agonists (eg, fentanyl) and under some circumstances will antagonize the actions of full agonists. The pure opioid antagonists are discussed in Chapter 17.
The opioid drugs mimic endogenous compounds. Endorphins, enkephalins, and dynorphins are endogenous peptides that bind to opioid receptors. These three families of opioid peptides differ in their amino acid sequences, anatomic distributions, and receptor affinities.
Opioid receptor activation inhibits the presynaptic release and postsynaptic response to excitatory neurotransmitters (eg, acetylcholine, substance P) from nociceptive neurons. The cellular mechanism for this action was described at the beginning of this chapter. Transmission of pain impulses can be selectively modified at the level of the dorsal horn of the spinal cord with intrathecal or epidural administration of opioids. Opioid receptors also respond to systemically administered opioids. Modulation through a descending inhibitory pathway from the periaqueductal gray matter to the dorsal horn of the spinal cord may also play a role in opioid analgesia. Although opioids exert their greatest effect within the central nervous system, opiate receptors have also been identified on somatic and sympathetic peripheral nerves. Certain opioid side effects (eg, depression of gastrointestinal motility) are the result of opioid binding to receptors in peripheral tissues (eg, the wall of the gastrointestinal tract), and there are now selective antagonists for opioid actions outside the central nervous system (alvimopan and oral naltrexone). The distribution of opioid receptors on axons of primary sensory nerves and the clinical importance of these receptors (if present) remains speculative, despite the persisting practice of compounding of opioids in local anesthetic solutions applied to peripheral nerves.
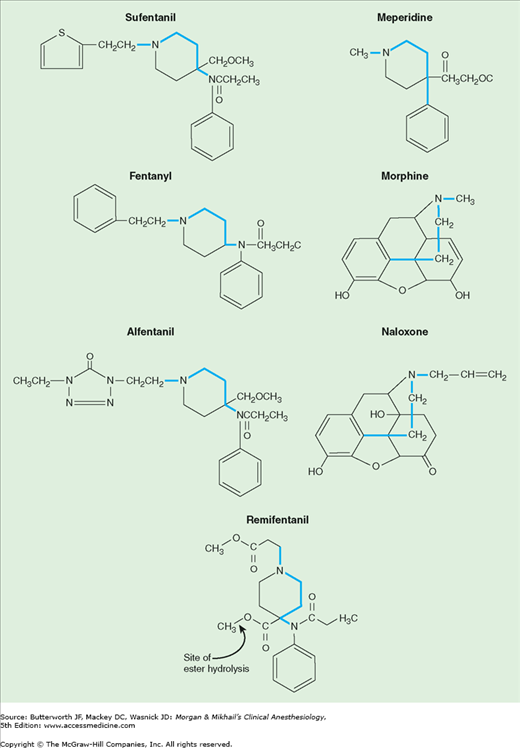
Opioid receptor binding is a property shared by a chemically diverse group of compounds. Nonetheless, there are common structural characteristics, which are shown in Figure 10-1. As is true for most classes of drugs, small molecular changes can convert an agonist into an antagonist. The levorotatory isomers are generally more potent than the dextrorotatory opioid isomers.
Rapid and complete absorption follows the intramuscular injection of hydromorphone, morphine, or meperidine, with peak plasma levels usually reached after 20-60 min. Oral transmucosal fentanyl citrate absorption (fentanyl “lollipop”) provides rapid onset of analgesia and sedation in patients who are not good candidates for conventional oral, intravenous, or intramuscular dosing of opioids.
The low molecular weight and high lipid solubility of fentanyl also favor transdermal absorption (the transdermal fentanyl “patch”). The amount of fentanyl absorbed per unit of time depends on the surface area of skin covered by the patch and also on local skin conditions (eg, blood flow). The time required to establish a reservoir of drug in the upper dermis delays by several hours the achievement of effective blood concentrations. Serum concentrations of fentanyl reach a plateau within 14-24 h of application (with peak levels occurring after a longer delay in elderly than in younger patients) and remain constant for up to 72 h. Continued absorption from the dermal reservoir accounts for persisting measurable serum levels many hours after patch removal. Fentanyl patches are most often used for outpatient management of chronic pain and are particularly appropriate for patients who require continuous opioid dosing but cannot take the much less expensive, but equally efficacious, oral agents such as methadone.
A wide variety of opioids are effective by oral administration, including oxycodone, hydrocodone (most often in combination with acetaminophen), codeine, tramadol, morphine, hydromorphone, and methadone. These agents are much used for outpatient pain management.
Fentanyl is often administered in small doses (10-25 mcg) with local anesthetics for spinal anesthesia, and adds to the analgesia when included with local anesthetics in epidural infusions. Morphine in doses between 0.1 and 0.5 mg and hydromorphone in doses between 0.05 and 0.2 mg provide 12-18 hours of analgesia after intrathecal administration. Morphine and hydromorphone are commonly included in local anesthetic solutions infused for postoperative epidural analgesia. Extended-release epidural morphine (DepoDur) is administered as a single epidural dose (5-15 mg), the effects of which persist for 48 h.
Table 10-2 summarizes the physical characteristics that determine distribution and tissue binding of opioid analgesics. After intravenous administration, the distribution half-lives of all of the opioids are fairly rapid (5-20 min). The low fat solubility of morphine slows passage across the blood-brain barrier, however, so that its onset of action is slow and its duration of action is prolonged. This contrasts with the increased lipid solubility of fentanyl and sufentanil, which are associated with a faster onset and shorter duration of action when administered in small doses. Interestingly, alfentanil has a more rapid onset of action and shorter duration of action than fentanyl following a bolus injection, even though it is less lipid soluble than fentanyl. The high nonionized fraction of alfentanil at physiological pH and its small volume of distribution (Vd) increase the amount of drug (as a percentage of the administered dose) available for binding in the brain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree