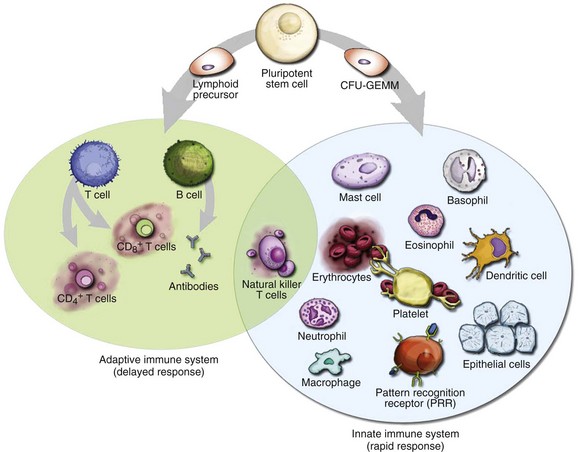
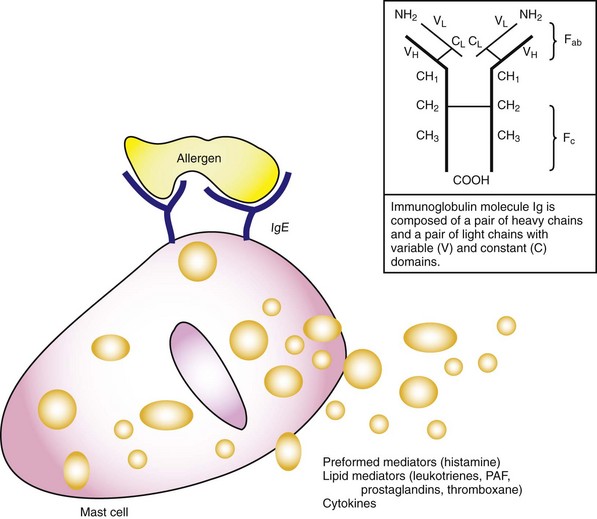
Chapter 119 The human immune system is an assemblage of cellular and humoral components working together in a highly complex, coordinated, and elegant fashion to achieve the primary goal of protecting the human host (self) from harmful offenders (nonself). Exposure to danger signals activates the various immune mechanisms to bring about immune responses aimed at neutralizing the dangerous nonself while preserving self.1 The immune system can, however, overreact to otherwise harmless nonself agents, producing inappropriate responses that are harmful to the host, thereby giving rise to allergy or allergic diseases. These hypersensitivity reactions are manifested in clinical symptoms ranging from mildly inconvenient to debilitating to fatal. For practical purposes, the term allergy is used in this chapter to refer to mast cell–mediated hypersensitivity reactions. For most allergic diseases to occur, predisposed individuals need to be exposed to allergens through a process called sensitization. Substances that elicit an allergic reaction are referred to as allergens, and those that elicit an antibody response (activated by B- and T-cell receptors) are called antigens. The incidence of anaphylaxis has not been determined with certainty, but recent evidence suggests that it is increasing.2 There are approximately 100,000 attacks of anaphylaxis in the United States per year, of which approximately 60,000 are first-time events and 1000 to 1500 are fatal.3 Factors affecting the incidence of anaphylaxis include time of the year, age, female sex (adults), higher socioeconomic status, northern locations, route of allergen exposure, and history of atopy (Box 119-1).2,4 Anaphylactic reactions seem to be more common in the summer and early fall, coincident with the outdoor season; in people of higher socioeconomic status; and in people with a history of atopy. In general, anaphylaxis is more common in adults, but in particular, it is more common in women older than 30 years and in boys younger than 16 years. The dose, frequency, duration, and route of administration of a drug also affect the tendency to develop an anaphylactic reaction; the parenteral route is more likely than the oral route to lead to an anaphylactic reaction. One interesting aspect of drug-related anaphylaxis is the constancy of administration. An anaphylactic reaction may not occur in an otherwise susceptible patient as long as a drug is administered at regular intervals. The same patient may, however, experience an anaphylactic reaction if the drug is resumed after an interruption of therapy.5 Risk factors for increased severity and mortality of an anaphylaxis reaction include having a recent episode of anaphylaxis, extremes of age, presence of atopy or cardiopulmonary conditions, taking medications that may influence timely recognition of the symptoms or impede the treatment of anaphylaxis, and rapid onset of symptoms after exposure (see Box 119-1).2,6 The more rapid an anaphylaxis reaction is after an exposure, the more likely it is to be severe and potentially fatal. Whereas anaphylaxis in an infant is a rare event, timely recognition of an anaphylactic reaction in an infant can be difficult because signs of anaphylaxis, such as flushing, vomiting and diarrhea, lethargy, and dystonia after feeding, can be overlooked as minor reactions.7 Elders, on the other hand, tend to have worse outcomes in anaphylaxis because of their comorbid conditions, notably heart failure, ischemic heart disease, hypertension, and obstructive lung diseases. A history of asthma and atopy is associated with a more severe or fatal anaphylactic reaction. This is particularly true when the allergen is administered by the mucosal route (e.g., food). Atopy does not, however, seem to be a risk factor when the allergen is administered parenterally (e.g., penicillin). Patients with psychiatric disorders and individuals taking medications and drugs that may impede prompt recognition of an anaphylaxis reaction are also at increased risks (e.g., recreational drugs, alcohol, tranquilizers, and hypnotics). In addition, two classes of medications concurrently taken by patients may increase their risks of anaphylaxis severity. ACE inhibitors can cause an accumulation of kinins and bradykinin and thus can exacerbate the angioedema in anaphylaxis. Beta-blockers can oppose the actions of adrenergic agents used in anaphylaxis treatment, potentially leading to protracted hypotension. Virtually any agent that is capable of activating mast cells (or basophils) can potentially precipitate an anaphylactic reaction. In approximately one third of the cases, however, an inciting agent cannot be identified.8 When a cause can be determined, foods, medications, and insect stings are the most common causes of anaphylactic reactions. Box 119-2 lists most common agents by their proposed immunologic mechanism.9 Reactions without identifiable causative agents are classified as physically induced or iatrogenic anaphylaxis. Foods are the major identifiable causative agents, accounting for approximately one third of the cases of anaphylaxis.8 A variety of foods have been identified, ranging from the well-known (nuts, shellfish, and eggs) to the obscure (chamomile tea, which may have cross-reactivity with ragweed). Cow’s milk, egg, peanut, soy, wheat, fish, shellfish, and tree nuts are foods that most commonly cause anaphylaxis. Even for a person with a known history of food allergy, it may be difficult to avoid foods that may cause allergic reactions because their identity may be obscured in processing (e.g., consuming wine contaminated with Hymenoptera venom).10 Because allergenic foods are first absorbed transmucosally, symptoms of food anaphylaxis may first appear localized to the upper airway of the respiratory tract. When anaphylactic allergens are administered parenterally, symptoms of anaphylaxis tend to be more cardiovascular and systemic. Allergic reactions to foodstuffs are more common in children, with incidence ranging from 0.3 to 7.5%.11 Therapeutic and prophylactic use of large quantities of antibiotics is common in the production of beef cattle, swine, fish, poultry, and sometimes vegetables and fruits. Along with antibiotics, sodium and potassium bisulfites and metabisulfites are used as preservatives in foods. Sulfites have been used as antioxidants in the food and restaurant industry to prevent discoloration of vegetables (e.g., salad bars and avocado dips), fruits, and potatoes and to preserve fruit and vegetable juices. They are also used to prevent bacterial contamination and oxidation of wines, beers, and distilled beverages. Sensitivity to ingested sulfites has been well documented, especially among asthmatics.12 Establishment of a particular foodstuff or preservative as the causative agent of anaphylaxis can be difficult. Allergic reactions to common antibiotics, such as benzylpenicillin, semisynthetic penicillin, and cephalosporins, are well documented; allergy to penicillin is perhaps the most commonly reported medication allergy. Because of their low molecular weights, these antimicrobials themselves do not possess antigenic properties (they are haptens). They become immunologically active (i.e., elicit an immunologic response) only after they bind to a “carrier” host protein. Although patients often report a history of penicillin allergy, this may not stand up to close scrutiny.13 Studies have shown that up to 90% of individuals with a reported history of penicillin allergy can safely use penicillin; these individuals usually either are mislabeled as penicillin allergic or lose their allergy after years of avoidance.14 Depending on the studies, the frequency of allergic reactions to penicillin varies from 0.01 to 0.05% (1 to 5 reactions per 10,000), with an anaphylactic reaction rate of less than 0.01% and a fatality rate of less than 0.002% (less than 1 fatality per 50,000 penicillin administrations).15 Parenterally administered penicillin is responsible for most of the anaphylactic reactions. The extensive use of this drug in unsuspected sources such as foods, in which it is used as a bacteriostatic agent, may make it difficult to ascertain historically that penicillin is not the causative agent. Cephalosporins share the β-lactam ring structure and side chains of the penicillins, and allergic cross-sensitivity has been incriminated in 1 to 8% of patients.16 Patients who have urticaria or anaphylactic reactions after taking penicillin are approximately four times more likely to have an adverse reaction to cephalosporins. Even in this setting, the risk of an anaphylactic reaction to cephalosporins is still less than 0.1%, and the first dose of cephalosporin can be administered orally under medical supervision.17 Hymenoptera venoms and fire ant stings are responsible for significant anaphylactic morbidity and mortality.18 The Hymenoptera venoms are complex mixtures of pharmacologically and biochemically active substances. Honeybee venom contains hyaluronidase, phospholipase A, and other peptides. Yellow jacket venom contains not only phospholipases A and B and hyaluronidase but also kinins. Hornet venom contains acetylcholine in addition to those typical peptides. Fire ant venom is mostly a nonproteinaceous alkaloid suspension containing phospholipase A and hyaluronidase. Stinging Hymenoptera insects affect up to 13.6 million Americans annually (c. 1999), accounting for approximately 50 to 100 deaths annually.19 Allergic sensitization to Hymenoptera has been reported in 0.4 to 4% of the general population. The principal offenders (in decreasing order of frequency) are yellow jackets, honeybees, wasps, and yellow and bald-faced hornets. The imported fire ant has become a significant pest responsible for anaphylaxis, spreading from the Atlantic and Gulf coasts inland.20 The introduction of killer (Africanized) bees in Brazil and their subsequent northern migration make them a significant cause of sting-induced anaphylaxis in areas of Texas, Arizona, and the southwestern United States.21 Latex and Medical Products.: Latex allergy refers to sensitivity to the proteins or chemicals contained in the latex products. The sensitivity reaction can be delayed (type IV) contact dermatitis or an immediate hypersensitivity (type I) reaction (Box 119-3). The most common symptoms of latex allergy include allergic urticaria, rhinitis, conjunctivitis, and occupational asthma. Although latex allergy used to be more common, with the institution of nonpowdered gloves and nonlatex gloves in hospitals, anaphylactic reaction from latex has become an uncommon event.22 Anaphylactic reactions have also occurred against ethylene oxide (ETO), which is used to sterilize hemodialyzers. ETO can bind with human proteins such as human serum albumin (HSA), rendering the ETO-HSA complex allergenic. Anesthetic Drugs.: Local anesthetics produce occasional adverse reactions.23 Most of these reactions are not allergic in nature but are related to a direct effect of the medication. True allergic reactions are uncommon and are most commonly seen with local anesthetics from the ester family (e.g., procaine, tetracaine, and benzocaine). Allergic reactions to local anesthetics belonging to the amide family (e.g., lidocaine, bupivacaine, mepivacaine, and dibucaine) are rare. Multidose vials of lidocaine contain the preservative methylparaben, which belongs structurally to the ester family. This preservative has been implicated in allergic reactions in patients with a history of previous lidocaine hypersensitivity.24 Only pure lidocaine (without the methylparaben preservative) should be used in intravenous applications (as in Bier’s block). Vaccines.: Anaphylactic reactions have occurred after the administration of egg embryo–grown vaccines, including the combined measles, mumps, and rubella (MMR), yellow fever, and influenza vaccines. Patients who are able to tolerate eggs orally are likely to tolerate the vaccines. Blood Products.: Anaphylactic-type reactions are an uncommon complication of the administration of whole blood and immunoglobulins. The fixation of antibodies to formed elements such as red blood cells, platelets, and leukocytes and soluble components activates the complement system. This is particularly relevant in IgA-deficient patients exposed to multiple transfusions, who may have produced antibodies to IgA in previous transfusions. With subsequent transfusions, an antigen (IgA)–anti-IgA antibody (IgG) immune complex forms, leading to subsequent activation of the complement cascade. Opiates.: Many of the opioid analgesics can cause anaphylactic reaction through a direct histamine release mechanism, although some are IgE mediated. It is unclear how much cross-sensitivity is present among these agents.25 Radiocontrast Media.: Radiocontrast media (RCM) represent an important class of drugs that can cause an anaphylactic reaction. Approximately 10 million radiologic studies using RCM are performed in the United States annually. Anaphylactic reactions to RCM are largely idiosyncratic, occur within minutes of infusion, and are independent of the dose. The pathophysiologic mechanism of anaphylactic reactions to RCM is unknown, but it is believed to be nonimmunologic. Suggested mechanisms include direct histamine release, alternative complement pathway activation, and activation of the contact system. Risk factors for an anaphylactic reaction include a previous adverse reaction to RCM, a history of atopy or allergic disease, asthma, and certain medications. A history of allergy to fish or shellfish is not a contraindication to the use of RCM, nor does it increase the risk of an adverse reaction to RCM.26 Clinically, the risk for severe adverse reaction is 0.16% with ionic contrast materials and 0.03% with nonionic contrast materials. The death rate from RCM reactions is estimated at 1 to 3 per 100,000 administrations of contrast material.27 Protocols have been developed to minimize risks of a serious allergic reaction in patients who have had a previous adverse reaction to RCM but who still require additional radiographic studies with contrast agents (Box 119-4). Aspirin and NSAIDs.: Aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs) are believed to cause anaphylaxis (or anaphylactoid reaction) through interruption of arachidonic acid metabolism.28 The incidence of anaphylaxis to aspirin and NSAIDs varies widely, depending on the population (healthy, atopic, or those with nasal polyps). One study estimated the incidence as 2.1 anaphylaxis cases per 100,000 exposed patients.15 Desensitization protocols have been suggested for cardiovascular patients with a history of aspirin allergy.29 For aspirin-induced cutaneous disease, an aspirin desensitization protocol that can be used for cardiovascular patients in the ED is to administer aspirin every 15 minutes, starting with 0.1 mg, up to 325 mg at 135 (FD&C Yellow No. 5), may also cause anaphylaxis through modulation of arachidonic acid metabolism.31 Immunotherapy Drugs.: Allergen extracts are commonly used in skin testing and in immunotherapy (also known as hyposensitization or desensitization).32 Exposure to therapeutic pollens, by injection or inhalation, can result in local allergic or systemic anaphylactic reactions. High-dose therapy, too frequent administration, or inadvertent intravascular injection increases the risk of anaphylaxis with immunotherapy. Steroids.: Although corticosteroids are used in the management of acute allergic syndromes and anaphylaxis, adverse reactions to these medications have been observed after parenteral administration.33 Skin testing may demonstrate the specific class of steroids responsible for hypersensitivity, and substitution of a different class should be considered. Exercise (Physical)–Induced Anaphylaxis.: Thermomechanical and physical factors (heat and cold), especially exercise, have increasingly been recognized as etiologic agents in certain anaphylactic-like incidents.34 The mechanism is unclear, but release of mediators from mast cells and basophils has been implicated. Patients with exercise-induced anaphylaxis are generally dedicated athletes who may have a personal or family atopic history. Exercise-induced anaphylaxis has been demonstrated in some cases to depend on previous ingestion of food to which the patient may be subclinically sensitive. Provocative foods, if identified, should be avoided. Patients should discontinue the exercise at the onset of rash or pruritus. When exercise is continued beyond this point, clinical deterioration is likely in susceptible individuals. Prophylactic treatment with an antihistamine as a single agent or in combination with other agents may be helpful. Avoidance of precipitating factors, modification of exercise, and use of a self-injectable epinephrine kit are recommended for patients with exercise-induced anaphylaxis. Idiopathic Anaphylaxis.: In the United States, approximately 20,000 to 47,000 patients annually see allergists for signs and symptoms of idiopathic anaphylaxis (IA).35 The diagnosis of IA is made only after extensive evaluation by the allergist. Although IA may be life-threatening, it is usually responsive to conventional therapies, including antihistamines, sympathomimetics, and especially prednisone.36 Some cases of IA may appear to be caused by the act of kissing but are in fact caused by food or conversion disorders.37 The overall prognosis for IA is good, but certain patients may experience recurrent IA despite intensive prophylactic therapy. Sometimes, IA can represent “progesterone” anaphylaxis.38 Women suffering from this disorder may present with recurrent episodes of anaphylaxis that are temporally related to the menstrual cycle. Other patients may have anaphylactic reactions to injection of medroxyprogesterone or luteinizing hormone–releasing hormone. Because allergy is intimately related to immunology, a brief review of immunology is included in this chapter. Immunologic responses to antigens in humans are coordinated by two systems: the ancient innate immune system, which humans inherited from invertebrates; and the recently evolved adaptive immune system, which is present in humans and vertebrates (Fig. 119-1). The innate immune system is considered the first line of defense, characterized by its nonspecific but rapid responses to offending agents or microbes. Its effector components include resident cells (epithelial cells, mast cells, macrophages, dendritic cells, antimicrobial proteins), infiltrative cells (natural killer cells, neutrophils, monocytes, dendritic cells), and various proteins (antimicrobial peptides, complements, cytokines, pathogenic pattern recognition receptor [PRR] system). Encoded in the germline, PRRs can recognize pathogen-associated molecular patterns (PAMPs) extracellularly or intracellularly. PAMPs are evolutionarily conserved molecular patterns that are present in microbes but not in humans (except in mitochondrial DNA).39 On exposure of the human host to these PAMPs, the PRR and subsequently the innate system and the inflammatory cascade are activated, leading to the clearance of dangerous PAMPs.40 The innate system responds to the danger signals rapidly and nonspecifically, whereas the adaptive immune system takes time for the antigen-specific cells (B and T cells) to amplify through a process known as clonal expansion to mount a specific immune response. In contrast to the innate system, the adaptive immune system is characterized by the delayed response, immune memory, enormous diversity, and exquisite specificity. The effector components of the adaptive system include B and T lymphocytes and cytokines. T and B cells are capable of recognizing myriad antigens through a vast library of antibodies and receptors (up to 1015). This diversity is accomplished by somatic rearrangement of fewer than 400 genes.41,42 The adaptive and innate immune systems originate from the common pluripotential hematopoietic stem cells, which are derived from the yolk sac and later reside in the bone marrow. These stem cells differentiate and develop into the lymphoid precursor cells and the colony-forming unit for granulocyte, erythroid, myeloid, and megakaryocyte (CFU-GEMM) stem cells. The lymphoid precursor cells differentiate into bursa-equivalent lymphocytes (B cells), thymus-derived lymphocytes (T cells), and natural killer cells. The CFU-GEMM cells develop into mast cells, basophils, and others (see Fig. 119-1). When the host encounters a foreign antigen, the cellular components of the adaptive immune system interact with the cellular and protein components of the innate immune system to mount a concerted defense aimed at neutralization of the antigen. Lymphoid precursor cells migrate from the bone marrow into the thymus, where they continue their ontogeny.43 Under regulation by cytokines and cell-cell interaction, these precursors undergo gene rearrangement and positive and negative selection. In the process, T cells acquire the T-cell antigen receptors and various surface markers. Two types of T cells mature and come out of the thymus: CD4+, also called helper T cells (60-70%), and CD8−, also called suppressor T cells (30-40%). Depending on the type of cytokine produced, T helper cells differentiate into type 1 helper cells (TH1) and type 2 helper cells (TH2), with opposing activities. Whereas TH1 cells inhibit IgE production and IgE isotype switching, TH2 cells stimulate IgE production and IgE isotype switching. The balance of these stimulatory and inhibitory activities of the TH1 and TH2 cells is believed to determine an individual’s propensity to develop allergic disease or atopy and may help explain the increased prevalence of allergy in urbanized and Western societies in the past three decades. Early in utero and soon after birth, naïve T lymphocytes in the infant’s immune system are dominated by the allergy-prone TH2 cells and their associated cytokines (interleukins 4, 5, and 13). These cytokines are important inducers for production of IgE antibodies. Later, during infancy through early childhood and adolescence, the nonatopic infant’s immune system gradually shifts from this allergy-prone TH2 environment to an allergy-protective TH1 environment. The cytokines associated with this TH1 environment include interleukin-2 and interferon-γ. This shift is thought to be caused by the continual exposure of the young individual’s immune system to allergenic stimuli from the surrounding environment, mainly microbes. Features of Western lifestyles, such as changes in infant diets, widespread use of antibiotics, smaller family size, and cleaner childcare, are believed to reduce this stimulatory antigenic exposure in an individual’s early years, leading to an environment in which the immune system is dominated by a persistent allergy-prone TH2 system (the hygiene hypothesis). This imbalance between the two immune systems supposedly ultimately leads to atopy and thus an allergy-prone population.44 B-cell ontogeny is divided into antigen-independent and antigen-dependent stages.43 During the antigen-independent stage, B cells mature in primary lymphoid organs (bone marrow and fetal liver), where they undergo gene rearrangement in a stochastic manner and acquire various surface markers. Later during the antigen-dependent stage in the secondary lymphoid organs (lymph nodes and spleen), B cells differentiate into memory B cells and plasma cells and are ready to secrete immunoglobulins. Throughout B-cell ontogeny, B-cell maturation, isotype switching, and immunoglobulin production are driven by activated T cells, cytokines, and interaction with antigen and bone marrow stromal cells. Immunoglobulins are protein molecules composed of two identical polypeptide heavy chains and two identical polypeptide light chains, covalently linked by disulfide bonds (Fig. 119-2). The heavy (H) chains have one variable domain, VH, and three or four constant domains, CH. The light (L) chains have one variable domain, VL, and one constant domain, CL. The variable domains of the heavy and light chains together form a pair of identical antigen-binding sites and with the adjacent constant heavy domain pair make up the Fab (antibody-binding fragment) region of the immunoglobulin molecule. The remaining constant domains of the heavy chains together form the Fc (crystallizable fragment) region of the immunoglobulin molecule. The Fc binds to the surface receptors of effector cells, such as mast cells, B cells, or macrophages. There are five isotypes or classes of immunoglobulins, IgG, IgA, IgM, IgD, and IgE; isotype IgG has four subclasses (IgG1, IgG2, IgG3, and IgG4), and IgA has two subclasses (IgA1 and IgA2). The body usually produces IgM antibodies when it first encounters an antigen. Repeated antigenic exposure, however, may cause the constant region of the IgM to switch to another class (IgA, IgG, or IgE), a process also known as isotype switching. Isotype IgE (and IgG4) is the most important antibody in the pathogenesis of allergic disease and anaphylaxis. The term allergy is commonly used to describe clinical illnesses produced by excessive immune responses by a normal immune system to otherwise innocuous allergens. In this chapter, we adapt the classic Coombs and Gell classification to categorize these hypersensitivity reactions (Box 119-5).
Allergy, Hypersensitivity, Angioedema, and Anaphylaxis
Perspective
Epidemiology and Risk Factors
Triggers for Anaphylaxis
Foods
Antibiotics
Insect Stings
Other Agents
Principles of Disease
Development of the Immune System and Mechanism of Immune-Mediated Injury
T-Cell Development
B-Cell Development and Immunoglobulins
Classification of Reactions
Allergy, Hypersensitivity, Angioedema, and Anaphylaxis