161 Alcoholic Ketoacidosis
• Alcoholic ketoacidosis accounts for up to 20% of cases of ketoacidosis.
• The characteristic example is an alcoholic person who abruptly abstains and has signs and symptoms such as vomiting, abdominal pain, malnutrition, and an anion gap metabolic acidosis, but no measurable alcohol levels.
• Initial glucose levels may be low, normal, or high.
• A ratio of β-hydroxybutyrate to acetoacetate in excess of 10 : 1 is pathognomonic for alcoholic ketoacidosis, whereas a 3 : 1 ratio is more common in diabetic ketoacidosis.
• Treatment emphasizes hydration with dextrose-containing solutions and thiamine; resolution of the acidosis usually occurs within 6 to 12 hours.
• Mortality from uncomplicated alcoholic ketoacidosis is less than 1%.
Definition and Epidemiology
The diagnosis of alcoholic ketoacidosis (AKA) is established when an alcoholic patient is found to have an anion gap metabolic acidosis without historical or laboratory evidence suggesting an alternative cause. AKA may develop after protracted vomiting in malnourished, chronic alcoholics who consume a daily average of 200 g of ethanol. AKA generally occurs with equal frequency in adult men and women between 20 and 60 years of age. Its incidence and prevalence remain undefined. Up to one half of patients are likely to suffer recurrence. It is unclear whether these individuals have a genetic predisposition to AKA or whether they repeatedly reproduce the hormonal milieu that precipitates ketoacidosis. Almost one fifth of cases of ketoacidosis are alcoholic ketoacidosis.1–3
Pathophysiology
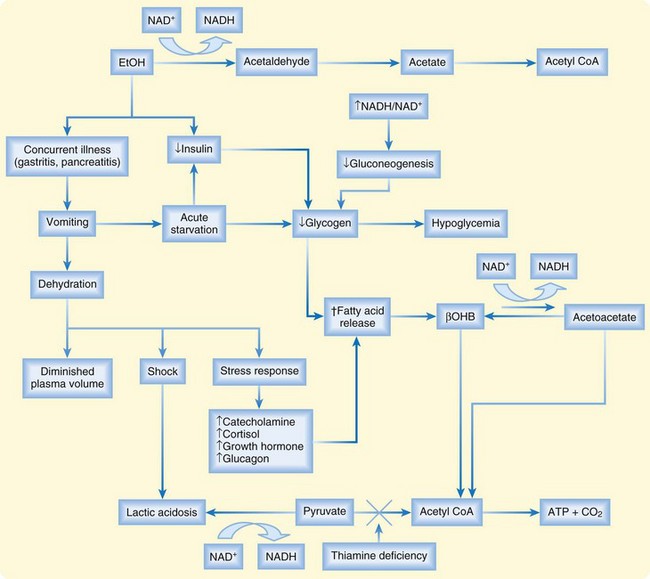
The term alcoholic acidosis describes a syndrome of four types of metabolic acidosis that occur in alcoholics and vary in severity: ketoacidosis, lactic acidosis, acetic acidosis, and loss of bicarbonate in urine. AKA arises from a complicated interplay of the metabolic effects of alcohol in fasted, dehydrated alcoholics who abruptly stop their intake of ethanol.4
β-Hydroxybutyrate is the predominant ketoacid.5 Metabolism of ethanol to acetaldehyde is catalyzed by alcohol dehydrogenase in the liver and results in accumulation of the reduced form of nicotinamide adenine dinucleotide (NADH) relative to the oxidized form of nicotinamide adenine dinucleotide (NAD+). The altered ratio of NADH/NAD+ is the rate-limiting step in alcohol metabolism and favors the conversion of acetoacetate to β-hydroxybutyrate, as illustrated in Figure 161.1.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree