Chapter 119 Airway Management
Anatomic Considerations
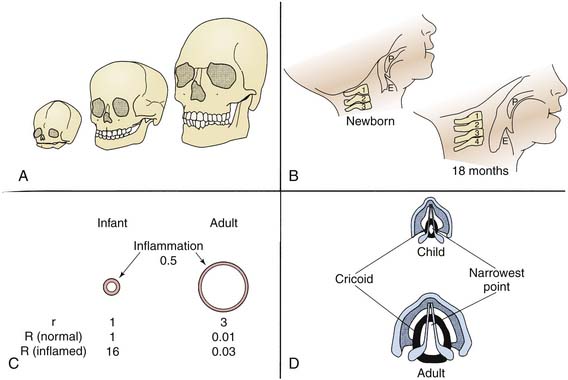
The configuration of the child’s airway changes dramatically from birth to adulthood (Figure 119-1). The nose is the site of nearly half of the total respiratory resistance to air flow at all ages. The infant’s nose is short, soft, and flat, with small, nearly circular nares. The nasal valve, the narrowest portion of the nasal airway, approximately 1 cm proximal to the alar rim in newborns, measures only about 20 mm2.1,2 By 6 months, dimensions of the nares have nearly doubled, but they are still easily occluded by edema, secretions, or external pressure. Although an infant is perhaps not as much the obligate nose breather as commonly assumed, signs of airway obstruction frequently develop when the infant’s nose is blocked.3,4
Under normal conditions, the genioglossus muscle and other muscles of the pharynx and larynx help maintain airway patency. Both tonic and phasic inspiratory activity synchronized with phrenic contraction have been noted in animal and human studies. In particular, the genioglossus increases the dimensions of the pharyngeal airway by displacing the tongue anteriorly.5 In the infant and young child, the tongue is large relative to the bony structures surrounding it and the cavities they form. Relatively little displacement is possible at any time, and loss of tone during sleep, sedation, or central or peripheral nervous system dysfunction is more likely than in older patients to allow the tongue to relax into the posterior pharynx and cause upper airway obstruction.
The internal dimensions of the trachea in a newborn are approximately one third those of an adult, and absolute resistance to air flow is higher in newborns than in older children and adults. Because the most important factor determining resistance (R) is the radius (r) of an airway (R proportional to 8 l/r4), small changes in airway diameter in infants or young children as a consequence of edema or secretions have a far greater effect on resistance than similar changes in larger patients (see Figure 119-1).
Basic Airway Management
Airway management depends on a brisk assessment of the patient’s breathing and knowledge of the likely progression of the airway problem, that is, deterioration versus improving function. In virtually any setting in which respiratory difficulty is suspected, oxygen should be administered until the specific abnormality can be identified and adequately treated. Although extreme hypercarbia usually is well tolerated, hypoxia is routinely catastrophic and is not necessarily obvious on initial examination. From the alveolar air equation, it is obvious that hypercarbia produces hypoxia at low fraction of inspired oxygen (FiO2) (Table 119-1). If the patient is breathing spontaneously, attention is directed first to signs of upper airway obstruction, including absence of audible or palpable air flow, stertorous sounds, stridor, or a rocking chest and abdominal motion rather than the normal, smooth rise and fall that should occur with inspiration and expiration.
Table 119–1 Impact of Providing Supplemental Oxygen During Hypercarbia on Alveolar Oxygen Tension
| Alveolar gas equation: | PaO2 = FiO2 (PB – Ph2o) – PaCO2/0.8 |
| Room air, normocarbia: | PaO2 = 0.21 (760 – 47) – 40/0.8 = 99 mm H2O |
| Room air, hypercarbia: | PaO2 = 0.21 (760 – 47) – 80/0.8 = 50 mm H2O |
| Supplemental O2, hypercarbia: | PAO2 = 0.4 (760 – 47) – 80/0.8 = 185 mm H2O |
FiO2, Fraction of inspired oxygen; PAO2, partial pressure of oxygen, alveolar.
Nasopharyngeal Airway
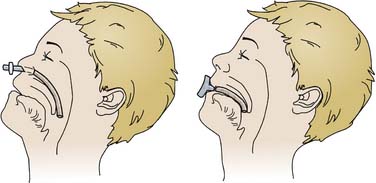
A nasopharyngeal airway that extends through nasal passages to the posterior pharynx and beyond the base of the tongue often is adequate to relieve obstruction and is tolerated by most patients, even those who are conscious (Figure 119-2). An appropriate-size airway extends from the nares to the tragus of the ear and is of the largest diameter that passes through nasal passages without causing blanching of the skin surrounding the nares. The airway tube should be well lubricated before placement. Risks of nasopharyngeal airways include nasal ulceration, bleeding, laceration of friable lymphoid tissue, rupture of a pharyngeal abscess, laryngospasm, and potential passage through the cribriform plate in patients with basilar skull fractures. Topical vasoconstricting agents reduce but do not eliminate the risk of bleeding. Like other nasal tubes, use of nasal airways increases the risk of sinusitis; therefore, contraindications to their use include severe coagulopathy, cerebrospinal fluid (CSF) leaks, and basilar skull fractures.
Oropharyngeal Airways
Oropharyngeal airways displace the base of the tongue from the posterior pharyngeal wall and break contact between the tongue and palate (see Figure 119-2). Size selection is important. An excessively long airway may encroach upon the larynx and cause laryngospasm. An airway that is too short may actually push the tongue posteriorly and exacerbate obstruction. If the airway is held at the side of the face with the flange just anterior to the incisors, the tip should be at or near the angle of the mandible. The airway should be positioned following the curve of the tongue while the tongue is held down and forward with a tongue depressor. Inserting the airway with its concave side facing the palate and then rotating it may traumatize the oral mucosa or damage teeth. Oral airways are poorly tolerated in any patient with a functional gag reflex and may induce vomiting. As a consequence, they are of little more than temporary value in the critically ill child. They may support a patent airway for bag-valve-mask ventilation in preparation for intubation.
Oxygen Delivery Devices
Nasal Cannulas
Nasal cannulas consist of two hollow prongs projecting from a hollow face piece. Humidified oxygen (100%) flows from a standard source, effectively delivering a pharyngeal concentration of 25% to 40% after mixing with variable amounts of room air. The cannulas are easy to use, often readily tolerated, lightweight, economical, and disposable and take advantage of the humidifying properties of the nasopharynx. Flow typically is limited to only 3 to 5 L/min because of the extent to which relatively dry gas flow cools and dries the nasal airway. The use of nasal cannulas is limited by the relatively low oxygen concentration that can be delivered. High-flow nasal cannulas can deliver up to 40 L/min of warmed, humidified gas and are generally very well tolerated, although the noise level for the patient can be high. The oxygen concentration delivered is higher than with simple nasal cannulas. High-flow nasal cannulas generate positive distending pressure similar to that provided by nasal continuous positive airway pressure. The pressure generated is dependent on the interaction among the flow rate, patient size, and anatomy of the patient’s airway, but it is probably limited to 4 to 5 cm H2O.6,7 At least in infants, positive pressure generation requires a closed mouth.8
Masks
Noninvasive Positive Pressure Ventilation
Both CPAP and BiPAP offer the advantage of providing respiratory support without endotracheal intubation but require that the child tolerate a close-fitting mask. A more extensive discussion of CPAP and BiPAP is available in Chapter 49, Mechanical Ventilation and Respiratory Care.
Establishing a Functional Airway
If the child is too weak or obtunded to maintain pharyngeal tone independently, the head should be placed on a thin cushion to cause slight cervical spine flexion and gentle extension at the atlantooccipital joint. In infants, the large occipitofrontal diameter makes the cushion unnecessary, although a thin pad under the shoulders may be useful. It appears that aligning the external auditory meatus with the sternal notch is a reasonable guide to appropriate positioning. Current recommendations are to avoid overextending the baby’s very flexible cervical spine, which may stretch and compress the trachea and potentiate, rather than relieve, obstruction. Studies have questioned the existence of this phenomenon but to date have included a very small number of infants, all with normal airways.9 Appropriate head tilt separates the tongue from the posterior pharyngeal wall. If airway obstruction persists, the chin can be pulled forward by encircling the mandible behind the lower incisors between the thumb and fingers. The most effective means of relieving functional obstruction is the so-called triple airway maneuver: With the fingers behind the vertical ramus of the jaw, the mandible is displaced downward, forward, and finally upward again until the mandible and lower incisors are anterior to the maxilla. This action effectively pulls the tongue forward and away from the pharyngeal wall.
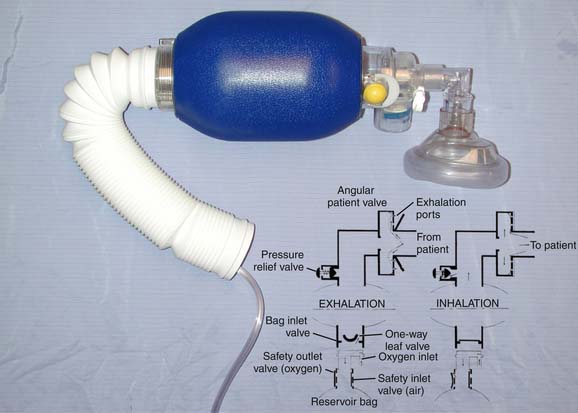
Two types of bags are in general use: self-inflating resuscitation bags and standard anesthesia bags. Because self-inflating bags vary substantially, specific directions for their use must be followed carefully. All bags incorporate an adapter to connect to a mask or endotracheal tube, a bag, a pressure-relief valve, and a port for fresh gas inflow. Most bags designed for children have pressure-relief valves designed to pop off at 35 to 45 cm H2O pressure to prevent excessive volume delivery and subsequent barotrauma. In patients with very poor compliance or increased airway resistance, it may be necessary to bypass this valve temporarily to provide effective ventilation. Most systems incorporate valves that prevent rebreathing. Fresh gas flows through the valve on spontaneous inspiration (negative pressure) or on creation of positive pressure by squeezing the bag (Figure 119-3). Exhaled gas is vented to the atmosphere. Not all systems allow spontaneous breathing; those that do demand that the patient generate at least a little negative pressure, so a good mask fit is necessary. Holding the mask above the patient’s face provides no supplemental oxygen. The percentage of oxygen delivered depends on the percentage of oxygen from the source, the fresh gas flow rate, and the respiratory rate, which determines the time available for the bag to refill. Most systems require some sort of reservoir assembly in addition to the self-inflating bag to prevent entrainment of room air. With a reservoir, 100% oxygen may be delivered; without a reservoir, most deliver less than 50%.
Anesthesia bags require flow from a source of gas under pressure in order to expand. Many variations have been reviewed extensively in the anesthesia literature. These circuits depend on the location of the fresh gas inflow and overflow valves, the rate of fresh gas flow, the respiratory rate, tidal volume, carbon dioxide production, and whether ventilation is spontaneous or controlled. Many ICUs use the Mapleson D configuration, with the fresh gas source attached just distal to the patient connection. The overflow valve is proximal to the reservoir bag. During expiration, the patient’s exhaled tidal volume mixes with fresh gas flowing into the system and accumulates in the tubing and bag. With sufficiently high fresh gas flow, alveolar gas is washed to the overflow valve and eliminated from the circuit. The system requires higher fresh gas flow to avoid rebreathing during spontaneous ventilation than during controlled breathing, but a safe rule of thumb recommends fresh gas flow two to three times the minute ventilation. During controlled ventilation, a minimum of 100 mL/kg/min ensures that carbon dioxide elimination is proportional to minute ventilation.10,11 At flows less than 90 mL/kg/min, increasing ventilation may only increase CO2 rebreathing.
Endotracheal Intubation
Indications
Respiratory Failure
Respiratory failure may result from dysfunction at any point along the ventilatory pathway. To provide appropriate support and to avoid hazards specific to the individual disorder, airway intervention must be tailored to the underlying cause. Outside the operating room, the need for intubation is most commonly associated with respiratory failure resulting from upper or lower airway or pulmonary parenchymal disorders that require mechanical ventilation. Respiratory failure is defined in terms of excessive work of breathing or inadequate oxygenation (in the absence of cyanotic congenital heart disease) or carbon dioxide elimination. Box 119-1 contains one set of criteria for intubation.
Hemodynamic Instability
Patients with hemodynamic instability often benefit from assisted ventilation. The need for controlled ventilation as a component of cardiopulmonary resuscitation is obvious. In addition, early intubation in anticipation of impending cardiovascular collapse may prevent catastrophic tissue hypoxia. Redistribution of blood flow away from respiratory muscles, especially the diaphragm, in patients with marginal cardiac output may improve perfusion of other vital organs, including the heart, and help prevent cardiac arrest.12–16
Neuromuscular Dysfunction
For additional information on neuromuscular dysfunction, see Chapter 58.
Neuromuscular dysfunction or severe chest wall instability (or deformity) may cause failure of the bellows apparatus for breathing.17 Initially, tidal volume remains normal or at least sufficient to maintain normal blood gas tensions, but vital capacity and maximal inspiratory and expiratory pressures decrease. Inability to take a deep breath or cough forcefully risks progressive segmental or lobar atelectasis, inability to clear secretions, bronchial obstruction, and possible major airway obstruction with sudden severe hypoxia or carbon dioxide retention. Increasing weakness results in progressively smaller tidal volumes, loss of upper airway tone, and, ultimately, inadequate minute ventilation. Bulbar dysfunction may lead to aspiration as a result of impaired swallowing and inadequate cough.
Measurement of ventilatory reserve provides a better assessment of the patient’s need for ventilatory assistance than does arterial blood gas tensions alone. Maximum negative inspiratory pressure and vital capacity are two simple, commonly used tests for this purpose. A variety of other measures also help assess respiratory “strength,” but most are difficult to perform in sick, uncooperative infants and children. Patients with diffuse neuromuscular weakness of any cause, spinal cord dysfunction above the level of T6, or loss of phrenic nerve or diaphragm function are particularly prone to respiratory failure.18 Because of the extreme compliance of their chest walls and relative ineffectiveness of intercostal muscles, infants younger than approximately 6 months tolerate diaphragmatic paralysis poorly.19–23
Many patients with neuromuscular weakness respond well to noninvasive forms of ventilatory support.17,24 Decisions about the best approach to airway management should be based on the nature and likely progression of the illness, the child’s maturity and level of consciousness, and the timing of the onset of respiratory insufficiency. In an emergency, endotracheal intubation is likely to be safest, with transition to noninvasive support when careful planning allows.24
Failure of Central Nervous System Regulation of Ventilatory Drive
Failure of central nervous system regulation of ventilatory drive may prompt intubation (see Chapters 54 and 57). Centrally mediated hypoventilation is manifest as CO2 retention, usually in the absence of increased work of breathing. On occasion the decision to support ventilation may be based on observing abnormal ventilatory patterns in anticipation of neurologic deterioration. Loss of protective airway reflexes, including the cough and gag reflexes, can result from central nervous system depression, cranial nerve abnormalities, or severe motor weakness. In such patients, intubation is indicated to prevent aspiration. Intubation may be appropriate in anticipation of the need to protect the airway and support ventilation during deep sedation for procedures or diagnostic studies.
Other Indications
Intubation is indicated as a step toward therapeutic controlled (hyper)ventilation (e.g., in patients with increased intracranial pressure [ICP] or pulmonary hypertension) or to support spontaneous hyperpnea in patients with metabolic acidosis and other conditions. Patients with profuse, thick, or tenacious secretions (e.g., as a result of bacterial pneumonitis or smoke inhalation) may benefit from an artificial airway as a means of providing effective suction. Impaired mucociliary clearance occurs in patients exposed to high oxygen concentrations or other airway irritants (including particulate and gaseous components of smoke), those experiencing severe hypoxia or hypercarbia, and, paradoxically, those who have airway trauma induced by endotracheal intubation and suctioning. Endotracheal intubation also provides an effective means of delivering drugs during cardiopulmonary resuscitation when venous or intraosseous access is not available (see Chapter 34).
Physiologic Effects of Intubation
Laryngoscopy is a potent physiologic stimulus (Box 119-2).25,26 At the very least, laryngoscopy is uncomfortable, causing significant pain and severe anxiety, especially in children who cannot understand or accept the need for it. Laryngoscopy causes an increase in systemic blood pressure and heart rate initiated by pressure on the back of the tongue or lifting the epiglottis.27 This effect is augmented by endotracheal intubation and suction.28 Nodal or ventricular dysrhythmias may occur. Sensory impulses triggering this reflex probably are carried along the vagus nerve supplying the base of the tongue, epiglottis, and trachea. The efferent limb is less well defined but most likely is the product of enhanced sympathetic activity. Infants respond more variably than do older patients. Hypertension develops in most patients, but a few become hypotensive, especially if they are hypoxic.29 They may demonstrate moderate-to-severe bradycardia rather than tachycardia, perhaps as a consequence of their greater parasympathetic tone. Sedation and light anesthesia decrease but do not obliterate the hypertension and tachycardia; surface anesthesia and deeper general anesthesia are more effective.30 Children with previous hypertension display an exaggerated vasopressor response. Sedation and neuromuscular blockade during airway manipulation in infants minimizes the associated bradycardia and systemic hypertension.31–35 The impact of positive pressure ventilation on cardiac performance depends on the underlying disorder (discussed in Chapter 26) but should be carefully considered in preparation for intubation.
During intubation, oxygen delivery to the patient is commonly interrupted. Ineffective breathing or apnea increases the likelihood of hypoxia, especially in children, with their relatively low functional residual volume and higher basal metabolic rate. Patients with severe pulmonary disease and abnormally low functional reserve capacity are at particular risk.31,34 During apnea, carbon dioxide tension increases at a rate of 3 to 4 mm Hg/min in healthy, sedated adults and probably more rapidly in children, particularly those with severe cardiopulmonary disease or increased metabolic rate resulting from fever, sepsis, or pain.36,37
ICP rises immediately during laryngoscopy even in patients without intracranial pathology before changes in blood gas tensions occur.30,32,38,39 Cerebral metabolic rate and blood flow increase. Hypoxia, hypercarbia, and diminished jugular venous drainage, particularly in struggling patients, contribute further to increases in cerebral blood volume and increased intracranial pressure. Although normally very transient, such intracranial hypertension may predispose patients with coagulopathies or vascular malformations to intracranial hemorrhage. Systemic hypertension in patients with impaired autoregulation of the cerebral circulation (e.g., sick infants or patients with a variety of intracranial disorders) and impedance to jugular venous return by jugular compression, pneumothorax, or coughing and struggling stress both the arterial and venous sides of the cerebral circulation. In patients with poor intracranial compliance, this effect is exaggerated and prolonged. In infants without primary central nervous system disease, muscle paralysis (even without sedation or analgesia) effectively blocks the rise in ICP associated with intubation.34 The systemic hypertensive response is generally unaffected by neuromuscular blocking agents but can be modified by analgesia and sedation or intravenous anesthesia.
Recognition of a Difficult Airway
Recognition of a difficult airway is important if potentially lethal surprises in airway management are to be minimized (Box 119-3). Although the intensivist is usually focused on the immediate physiologic disturbances affecting the patient, careful preparation and as thorough an evaluation of each individual patient as is possible is critical. Key components of the history and physical examination, as well as the clinical scenario, can provide insight into potential problem airways. A history of difficult intubations in the past or episodes of upper airway obstruction (including snoring or sleep apnea) suggest structural abnormalities that may or may not be evident at the moment. Recent tonsillectomy and adenoidectomy, cleft palate repair, or any prolonged surgical procedure resulting in oral edema or bleeding increase the likelihood of difficulty. Examining facial structure is essential, and inspecting the child’s profile is particularly important because significant abnormalities may not be fully apparent on frontal view alone (Figure 119-4). Certain genetic syndromes are associated with craniofacial anomalies, midline defects, or neuromuscular disorders that may make successful intubation via standard techniques exceptionally difficult. Treacher Collins syndrome (mandibulofacial dysostosis), Goldenhar syndrome (oculoauriculovertebral dysplasia), Down syndrome, Pierre Robin syndrome, and the mucopolysaccharidoses, such as Hurler syndrome and Hunter syndrome, are a few of the syndromes that have characteristic features that suggest the high probability of facing a challenging airway. Isolated micrognathia, macroglossia (glossoptosis), facial clefts, midface hypoplasia, prominent upper incisors or maxillary protrusion, facial asymmetry, a high arched narrow palate, a small mouth, and a short, muscular neck or morbid obesity are features that can interfere with effective bag-mask ventilation or visualization of the larynx. Limited temporomandibular joint or cervical spine mobility may make laryngoscopy and tube placement very difficult. Midface instability or upper airway bleeding, edema, airway or neck masses, and foreign bodies are additional reasons for concern.40
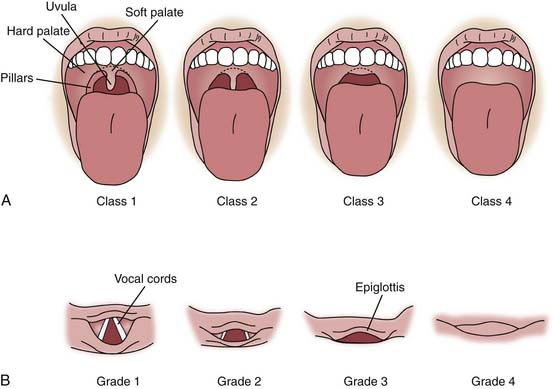
Several classification systems assist with recognition and classification of the adult patient with a difficult airway. Although never validated in pediatrics, they provide a useful framework for assessing infants and children. The Mallampati classification (Figure 119-5) assesses visualization of upper airway structures prior to intubation— particularly the uvula, soft palate, and faucial pillars—as a guide to the likely ease of intubation. Mallampati class 1 allows visualization of the uvula, soft palate, and faucial pillars; in class 2, faucial pillars and soft palate are visualized but the uvula is obstructed by the base of the tongue; in class 3 only the soft palate is visualized; and in class 4 the soft palate is not seen. Difficult intubation is more likely associated with classes 3 and 4. This scale can be used with cooperative children, and an approximate evaluation may be obtained by observing many crying infants and young children.
The Cormack laryngeal view grade score is shown in Figure 119-5, B. Because the Cormack system requires an attempt to visualize the larynx, it is more valuable as a tool for describing difficulty once it has been encountered than for predicting it. The Cormack score is grade 1, full view of vocal cords and glottis; grade 2, partial view of vocal cords and glottis; grade 3, only the epiglottis is seen; and grade 4, the glottis and epiglottis are not visualized. Grades 3 and 4 predict difficult direct laryngoscopy.41 Although these classification systems are helpful in a controlled environment, particularly the preoperative area of a hospital, they are recognized as having limited utility in the emergent situations often encountered in the ICU, and their ability to predict the degree of difficulty with intubation in children is not well established.42
Ability to visualize the faucial pillars, soft palate, and uvula usually predicts an uncomplicated intubation, but this may be difficult to assess in a sick, uncooperative child.43 Children with severe hypoxia, severe hypovolemia, or other causes of hemodynamic instability, such as intracranial hypertension, a full stomach, or some combination of these conditions, present added difficulties that must be considered.
When airway problems are anticipated, the intensivist should approach intubation with a plan specific to the difficulty noted and with a backup strategy in mind.44,45 Extra equipment should be on hand, including a variety of laryngoscope blades, forceps, tubes, bronchoscopes, tracheostomy or cricothyrotomy trays, and additional skilled personnel as needed.46 If sedation is required, agents that can be reversed pharmacologically are desirable and should be titrated slowly to the desired effect. Figure 119-6 shows a modification of the American Society of Anesthesiologist’s difficult airway algorithm and provides an approach to managing the difficult airway.47 A similar plan is necessary at the time of extubation, with serious consideration given to extubation in the operating room or with an airway exchange catheter left in place to facilitate reintubation if necessary.
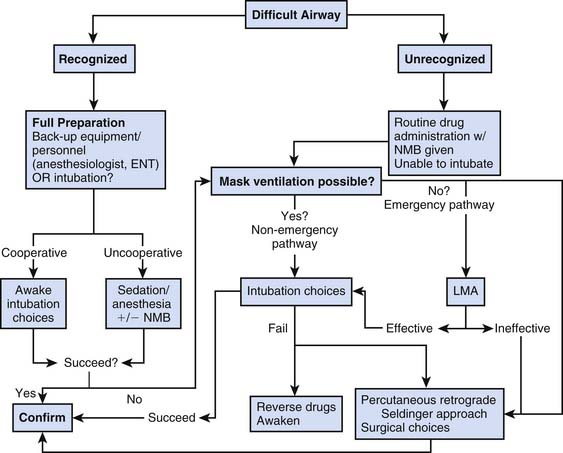
Figure 119–6 Modification of the American Society of Anesthesiologist’s difficult airway algorithm.
(From Practice guidelines for the management of the difficult airway: an updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway, Anesthesiology 98:1269, 2003.)
Process of Intubation
All equipment for intubation must be available prior to the procedure (Figure 119-7). A source of suction and appropriate catheters, oxygen and necessary tubing, ventilation bag, mask, laryngoscope and proper-sized blade with a well-functioning light, endotracheal tubes of the expected size and larger and smaller sizes, airway forceps, stylet, and a means of securing the endotracheal tube should be present at the head of the bed so that the intubator does not need to look away from the patient. A functioning intravenous catheter for drug infusion is essential in all but the most extreme emergencies.
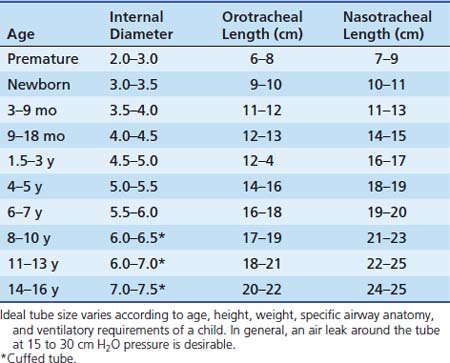
Selecting the proper tube size (diameter) is important, both to achieve effective mechanical ventilation and to prevent tracheal injury. A variety of formulas are in use, with the most common being that of Cole: Tube size (inner diameter) = (Age [years]/4) + 4. For infants, no formula is very accurate. Table 119-2 gives reasonable guidelines. Individual differences require that the tube size be modified for each child so that the tube passes easily and allows gas to leak around it at roughly 15 to 30 cm H2O pressure but fits snugly enough to allow delivery of adequate mechanical breaths at a given chest compliance.
Traditional teaching has held that use of cuffed endotracheal tubes is not necessary or appropriate in young pediatric patients (younger than 8 years) because the narrow diameter of the trachea at the cricoid ring allows a fairly snug fit without a cuff, and a cuff may make tracheal injury at that level more likely. In addition, the bulk of the cuff usually requires using a tube of 0.5 mm smaller diameter, with the associated increased resistance to gas flow and greater risk of occlusion. Cuffed tubes are routinely recommended for children older than 8 to 10 years because the cricoid ring has been replaced by the triangular opening of the vocal cords as the narrowest point in the airway (see Figure 119-1). In addition, the greater elastic recoil of the lungs and chest wall of older patients may demand higher airway pressures for effective ventilation.
However, cuffed tubes of all sizes are available and may be useful in patients in whom consistent minute ventilation is essential (e.g., in the presence of severely elevated ICP or very reactive pulmonary vasculature) or those requiring relatively high airway pressures. Although data are limited, evidence is growing that cuffed tubes can be used in young children without higher incidence of airway complications.48–52 The modern low-pressure, high-volume cuff requires a much lower pressure to obtain a seal than did the endotracheal tube cuffs of the past. When a cuffed tube is used, great care should be taken to inflate it with the “minimum occlusive volume,” the minimum volume required to “just seal” the gas leak around the tube during mechanical inspiration and prevent mucosal ischemia and subsequent tracheal damage. Potential advantages of cuffed tubes include decreased likelihood of multiple intubations to identify the correct size and avoidance of changing the endotracheal tube of a critically unstable patient if lung disease worsens. In addition, absence of a significant leak around the endotracheal tube (ETT) may decrease the likelihood of flow-triggered ventilator autocycling. The ability to occlude the leak also facilitates pulmonary function testing and indirect calorimetry.
Pharmacologic Agents Facilitating Intubation
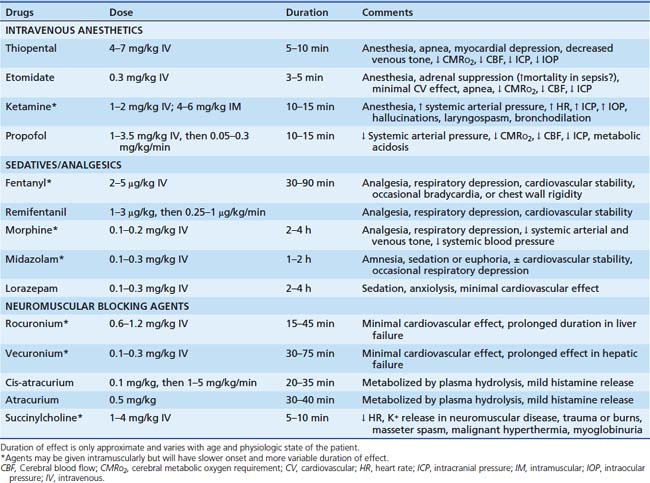
Although intubation often is possible without use of drugs, the physiologic and psychological benefits of their use usually outweigh the disadvantages.53,54 Analysis of data in the pediatric National Emergency Airway Registry shows that intubation success rate is higher when both sedation and neuromuscular blockade are used.55 This finding is equally true in neonates, in whom sedation and neuromuscular blockade are still commonly not used, with no evidence they are harmful.56 In neonates the predominance of evidence indicates that use of neuromuscular blockade is associated with a lower risk of intracranial hemorrhage and pulmonary airleak.57 Excellent technical airway skills are an absolute prerequisite, however, because loss of control of the airway invites catastrophe. Drugs facilitating intubation are listed in Table 119-3.
Sedative and Analgesic Agents
Etomidate is another short-acting intravenous anesthetic that causes rapid loss of consciousness (at 0.3 mg/kg) and respiratory depression, although it is less potent than thiopental. It also decreases cerebral oxygen consumption, CBF, and ICP, but without significant detrimental effects on cardiovascular function and with less respiratory depression than thiopental. These characteristics have led to its increased use for emergency intubation.58,59 It has no analgesic properties and may be best combined with a narcotic analgesic. Adverse effects include vomiting, myoclonus, and lowering of the seizure threshold. With continuous infusion for sedation, it can cause adrenal insufficiency, making it inappropriate for long-term use in the ICU.60 Growing evidence suggests it may suppress adrenal function even after a single dose, particularly in patients with sepsis and shock, raising questions about its use in these settings.61–70
Ketamine is another potent nonnarcotic analgesic and anesthetic that has been used safely in children in the critical care setting.71,72 It increases heart rate, systemic blood pressure, and cardiac output and is a fairly potent bronchodilator. However, myocardial depression may be apparent after administration to patients with catecholamine depletion. It may be of particular value in patients with status asthmaticus or other reasons for bronchospasm and may have a beneficial effect in sepsis. Spontaneous ventilation is preserved in most patients, but laryngospasm may occur.73 Although in the past it has been considered inappropriate for patients with intracranial hypertension because of evidence that it increases cerebral metabolic rate, blood flow, and ICP, more recent studies indicate that it may be used safely in these patients, although no clear consensus has emerged.74 Emergence delirium and hallucinations occur frequently and may be prolonged and recurrent, particularly in adolescents and young adults. Use of ketamine for a variety of procedures in children has been successful, with little reported difficulty with neuropsychiatric complications, but follow-up in most studies has been short and superficial.75–80 Whereas the majority of patients do not suffer severe disturbances, those who do may have severe and prolonged distress. Benzodiazepines or barbiturates may decrease the incidence and severity of such adverse effects and the incidence of vomiting, although the data in children are limited and conflicting.75,81,82
Propofol is an ultra-short-acting agent with rapid onset and offset unless given by continuous infusion. It causes respiratory depression, desaturation, and systemic hypotension secondary to its negative inotropic effects and peripheral venous and arterial vasodilation. Its role in airway management of critically ill children is limited because of these effects. It has gained widespread acceptance as an anesthetic agent in children, however, and has been used extensively for procedural sedation.83,84 Use in the ICU for more than approximately 6 hours is not recommended because of its still unexplained association with metabolic acidosis, cardiovascular collapse and death, propofol syndrome, among pediatric ICU patients.85–87 Current labeling warns against its use for prolonged sedation in children.
Narcotics commonly used for intensive care include morphine, fentanyl, and some of the ultra-short-acting agents such as remifentanil. They cause respiratory depression in a dose-dependent fashion and increase intracranial blood flow in proportion to the increase in PaCO2. If hypercarbia is prevented, they decrease cerebral metabolic rate and blood flow. In the setting of altered cerebral autoregulation, they may not protect the patient from alterations of CBF.88,89 Morphine causes histamine release and peripheral vasodilation and may precipitate systemic hypotension. Fentanyl is approximately 100 times more potent than morphine but does not release histamine and has little hemodynamic effect, even at anesthetic doses. Large doses given rapidly can cause bradycardia or chest wall rigidity. Remifentanil is a rapid-onset, ultra-short-acting opiate that is even more potent than fentanyl and may have potential benefit in intubation for procedures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree