72 Acute Parenchymal Disease in Pediatric Patients
 Diseases of the Airways
Diseases of the Airways
Status Asthmaticus
Although unusual anatomic conditions of the lower airways can occur in pediatric patients (Table 72-1), status asthmaticus and bronchiolitis are probably the most common causes of lower-airway disease encountered in the pediatric ICU. Asthma is common in the industrialized world, and the overall mortality rate attributable to asthma in the United States is estimated at 2.6 deaths per million children per year.1 Recurrent hospitalizations, previous ICU admissions, and the need for mechanical ventilatory support have been identified as risk factors for death from asthma.2 Status asthmaticus is characterized by acute, severe airway obstruction due to bronchoconstriction that is refractory to initial management with supplemental oxygen, inhaled bronchodilators, and corticosteroids. The pathophysiology of this condition begins with a precipitant that triggers contraction of hyperresponsive bronchial smooth muscle, mucus secretion, and mucosal edema, all of which lead to the obstruction of large and small airways (Figure 72-1). Hyperinflation from airflow limitation and premature closure of lower airways in expiration leads to increased end-expiratory lung volume3 and an increased respiratory workload, which ultimately set the stage for alveolar hypoventilation and hypoxemia. An abrupt and profound acidosis can develop when respiratory compensation for accumulated inorganic acids ceases to occur.3 On physical examination, the child with status asthmaticus can appear anxious or lethargic, will often demonstrate accessory muscle use, and depending on the quality of air entry, can demonstrate either cough with profound inspiratory and/or expiratory wheezing and prolongation of audible expiration, or a silent chest. An exaggerated pulsus paradoxus can often be demonstrated, a finding that reflects the profoundly negative intrapleural pressures generated by these patients during spontaneous respiration.
TABLE 72-1 Anatomic Causes of Lower-Airway Dysfunction
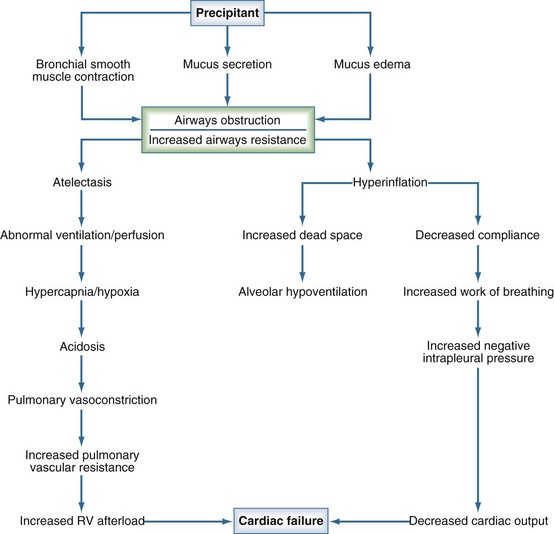
Figure 72-1 Pathophysiology of status asthmaticus.
(Modified from Helfaer M, Nichols D, Rogers M. Lower airway disease: bronchiolitis and asthma. In: Rogers M, editor. Textbook of Pediatric Intensive Care. 3rd ed. Baltimore: Williams and Wilkins; 1996, p. 141.)
Therapy
Supportive therapy for status asthmaticus begins with maintaining the airway, monitoring the quality of respirations, and ensuring euvolemia. Standard medical therapies for these patients include bronchodilators and corticosteroids, and several adjunct therapies have been investigated as possible rescue agents in difficult cases (Table 72-2). Short-acting β-agonist agents, which mediate airway smooth muscle relaxation via local β2 receptors,3 are the most commonly used bronchodilators for status asthmaticus. Among these agents, albuterol is the most widely used. Unlike epinephrine and isoproterenol, albuterol is relatively β2 selective,3 and it is most commonly administered by nebulization. It is typically given at a dose of 0.15 mg/kg (up to 2.5 mg/dose) on a frequent intermittent basis, but only a small fraction of the nebulized dose may actually be delivered to the lung, particularly in critically ill infants and children who are intubated with small tracheal tubes.4–6 Several studies have demonstrated that small doses of nebulized β-agonist given in rapid sequential fashion produce sustained improvements in forced expiratory volume more often than when larger doses are given less frequently,7,8 and there is also evidence to suggest that continuous nebulization of the drug may actually lead to more rapid and sustained clinical improvement.9
TABLE 72-2 Selected Pharmacotherapies for Status Asthmaticus
| Nebulized Therapies | Albuterol (0.5%), 0.15 mg/kg/dose (0.03 mL/kg/dose) inhaled q 1-6 h as needed (PRN) Continuous inhalation 0.5 mg/kg/h |
| Ipratropium, 0.25-0.5 mg inhaled q 4-6 h | |
| Racemic epinephrine (2.25%), 0.25-0.5 mL inhaled q 1 h PRN | |
| Subcutaneous (SQ) Therapies | Epinephrine (1 : 1000), 0.01 mg/kg/dose (0.01 mL/kg/dose) SQ (max 0.5 mL/dose) |
| Intravenous (IV) Therapies | Terbutaline, 10 µg/kg IV × 1, followed by 0.4-6.0 µg/kg/min IV infusion |
| Magnesium sulfate, 25-50 mg/kg IV over 20 minutes (max 2 g/dose) | |
| Methylprednisolone, 1 mg/kg/dose IV q 6 h |
In recent years, a preparation of the therapeutically active isomer of albuterol (levalbuterol) has become available. Levalbuterol appears to be effective when administered to children with stable asthma.10 There are no controlled trials presently available to evaluate its use in children with acute exacerbations of the disease. Inhaled anticholinergic agents such as ipratropium also have a role in the management of severe bronchospasm in children with asthma. Addition of inhaled ipratropium to inhaled β-agonists has been associated with favorable changes in pulmonary function, especially in children with severe asthma.11,12 For patients who do not respond to inhaled bronchodilators, it is possible to administer β-agonist therapy intravenously (IV). In some countries, the IV preparation of albuterol is available, which allows for an alternative administration route for this β2-selective agent. In the United States where IV albuterol is not available, terbutaline, which has some β2 selectivity, is a reasonable alternative. Although terbutaline has not been associated with clinically significant cardiac toxicity in most pediatric patients,3,13 many clinicians advise monitoring the electrocardiogram (ECG) and serum troponin level during its administration.
For as long as the inflammatory basis for asthma has been recognized, corticosteroids have had an important role in the management of status asthmaticus. The use of corticosteroids has been demonstrated to significantly improve airways obstruction in patients with severe acute asthma.14 The parenteral route is the method of choice for administering these agents to the critically ill child, and it is important to understand that fatal anaphylaxis to these drugs has been reported.15,16 Methylprednisolone is one of the most commonly used agents for acute severe asthma. Because of its half-life, steady-state levels can be achieved relatively quickly, and although dosing regimens vary, it is probably most appropriate to dose the drug every 6 hours. There does not seem to be any advantage to administering massive doses of glucocorticoids in status asthmaticus.17 If methylprednisolone is not available, equipotent doses of another glucocorticoid may be used.
Magnesium has been investigated for use in status asthmaticus because of its potential to augment the effects of bronchodilators by causing relaxation of airway smooth muscle. A recent randomized controlled trial in adults demonstrated that 2 g IV magnesium sulfate improves pulmonary function when administered as an adjunct to nebulized β-agonists and IV corticosteroids in patients with especially low forced expiratory volume in the first second of expiration (FEV1) (<20% of predicted).18 Although magnesium is occasionally added to standard therapy in pediatric status asthmaticus, the evidence supporting its use in this population is limited.19
Enthusiasm for the use of methylxanthines (theophylline, aminophylline) in pediatric asthma has fluctuated over time. These drugs act primarily as phosphodiesterase inhibitors, but the mechanism of their effects in asthma is not well understood. A recent randomized controlled trial investigated the effects of aminophylline in 163 children with status asthmaticus. Aminophylline was administered to these children as an adjunct to nebulized β-agonists, nebulized anticholinergics, and parenteral corticosteroids.20 The results of this trial suggested that aminophylline improved pulmonary function and may have averted intubation in a portion of those patients who received it.20 Although aminophylline may have a role in the treatment of severe status asthmaticus that is not responding to standard therapies, the potential for its widespread use is limited by its narrow therapeutic index.3
Bronchiolitis
Bronchiolitis is a clinical term implying an invasion of the large and small airway respiratory epithelium by inflammatory cells in the setting of acute respiratory illness. The primary cause of bronchiolitis is respiratory syncytial virus (RSV), which is responsible for 45% to 75% of cases, although parainfluenza viruses, rhinoviruses, adenoviruses, influenza viruses, enteroviruses, and Mycoplasma pneumoniae can produce the syndrome as well. RSV dependably produces yearly epidemics occurring during the winter and spring months. Infection with RSV is nearly universal among infants and children by 2 years of age. Although hospitalization rates vary seasonally and regionally, a recent study cited an average hospitalization rate between 3 per 1000 among children younger than 5 years of age, and 17 per 1000 among children younger than 6 months.21 Among all hospitalized children, the percentage requiring intensive care has been reported as 7% to 9% among patients without comorbidity and as high as 20% to 37% in those with preceding cardiac disease, chronic lung disease, prematurity, immunocompromise, and age younger than 6 weeks.22 Patients with these coexisting conditions are also at increased risk of mortality from RSV23 and have been identified as candidates to receive monthly prophylaxis with an RSV antigen–specific monoclonal antibody (Palivizumab [MedImmune Inc., Gaithersburg, Maryland]) during RSV season. However, recent epidemiologic data indicate that most RSV-infected children have no significant comorbidities, suggesting that prevention strategies targeting only medically complex patients may have minimal impact on the overall disease burden.21
RSV transmission can occur either by direct contact with contagious secretions or by exposure to aerosolized particles from the respiratory mucosa.24 The incubation period varies from 2 to 8 days,24 symptoms tend to escalate over 3 to 5 days, and convalescence can be prolonged up to several weeks in the most vulnerable small infants. On histologic examination, reappearance of ciliated respiratory epithelium commonly takes more than 2 weeks.24 Viral shedding from the respiratory tract typically occurs over 3 to 8 days but may also continue for up to 4 to 6 weeks in small infants. Symptoms typically begin with signs of upper respiratory illness, including fever, coryza, and possibly otitis media. Small infants commonly present with lethargy and central apnea25 early in the course of illness. Cough and tachypnea soon develop as the illness progresses to the lower airways, usually 1 to 3 days following incubation.24 Wheezing produced by flow limitation in peripheral airways is a nearly universal finding and may be due in large part to intermittent obstruction of large and small airways with necrotic epithelial debris, edema, and mucus24 rather than to the bronchospasm more commonly seen in asthma. Radiographic findings are often nonspecific but commonly include hyperinflation, peribronchial thickening, subsegmental consolidation, and multiple areas of atelectasis or infiltration involving most frequently the right middle and right upper lobes. A large prospective study of RSV-infected hospitalized children found that secondary bacterial infection occurred in only 1.2% of the study cohort, establishing that risk of bacterial disease is low in RSV bronchiolitis, despite potentially suggestive radiographic findings and the widespread empirical use of broad-spectrum antimicrobial agents in these patients.26
Therapy
Treatment of the infant or child with bronchiolitis is primarily supportive. Many years of clinical experience with empirical use of symptomatic medical therapies have failed to determine a clear role for any of these agents in the management of this disease. Data on the use of medical therapies in critically ill children with bronchiolitis is especially scant. Aerosolized ribavirin, a synthetic guanosine analog with broad-spectrum antiviral activity, is currently the only specific therapy approved for hospitalized infants with RSV bronchiolitis.24 In general, it has been shown to improve oxygenation and clinical status scores and reduce inflammatory mediators associated with ongoing wheezing in patients with RSV.24 A meta-analysis of three studies on the use of ribavirin in ventilated patients showed a small but significant decrease in ventilator days associated with the use of this agent.27 Nonetheless, prospects for widespread administration of this agent or even additional large-scale trials to further evaluate its role are limited by the technical challenges, cost, and occupational hazards associated with its use.28–30
Widespread use of bronchodilators and corticosteroids for the management of bronchiolitis is common despite the absence of evidence for improved clinical outcomes in critically ill children.27 There are presently no randomized controlled trials that have evaluated the efficacy of bronchodilators in critically ill children with bronchiolitis.31 Moreover, a recent large randomized controlled trial,32 as well as a systematic review,33 have failed to establish that any bronchodilator produces a significant improvement in relevant outcome measures in less severely ill hospitalized children with bronchiolitis. A few small studies have associated some short-term physiologic benefit with the use of corticosteroids and immune globulin in critically ill infants and children with bronchiolitis, but the efficacy of these therapies in altering outcomes in this population remains unproven.27 Following from the observation that critically ill children with severe bronchiolitis demonstrate surfactant deficiency and dysfunction, a great deal of interest surrounds the use of exogenous surfactant to modify the course of bronchiolitis in intubated patients. A number of small underpowered trials have been conducted on this topic,34–36 but the available data are not sufficient to provide a reliable estimate of surfactant’s effects in this setting.37 Moreover, the interpretation of this literature is complicated by the fact that the choice of surfactant preparation, the dosing regimen, and the mechanical ventilation strategy vary across studies, and each of these could have an important effect on outcome.37 An ongoing multicenter randomized controlled trial evaluating the impact of the synthetic surfactant, lucinactant (Discovery Laboratories, Warrington, Pennsylvania), on duration of mechanical ventilation among children younger than 2 years of age with acute hypoxemic respiratory failure38 may provide additional insight into surfactant’s therapeutic role in critically ill patients with bronchiolitis. Because future prospects for providing lasting immunity to RSV remain doubtful,24 there is an ongoing need for large multicenter studies to identify therapies which may benefit critically ill children with this disease.
Mechanical Ventilation
The need for mechanical ventilation in the patient with lower airways disease commonly arises from failure of ventilation and resulting hypercapnia. Hypoxemia and recurrent apnea, which are common in young infants with bronchiolitis, also frequently precipitate the institution of ventilatory support. Assuming adequate airway protection, oxygenation, and respiratory drive, it is probably best to avoid intubation in the patient with lower airways disease unless the overall clinical status of the child warrants the risk of augmenting airway hyperreactivity through airway instrumentation.39 To this end, there are several adjunct therapies that may obviate the need for intubation when added to aggressively applied conventional therapies. An inspired mixture of helium and oxygen (heliox) has been used to alleviate airflow limitation in pediatric patients. Owing to its low density and reduced Reynolds number, helium is able to convert turbulent gas flow to laminar flow in airways, and its clinical effect is generally immediate. Because it is an inert gas, it can potentially lower airway resistance without toxicity. When given as 60% to 80% of the total inspired gas mixture, helium can produce more efficient delivery of oxygen as well as nebulized drugs.40
The use of heliox in patients with lower-airway disease has generally produced inconsistent results. A small randomized controlled trial in spontaneously breathing children with status asthmaticus demonstrated that administration of heliox improves respiratory mechanics by lowering the pulsus paradoxus, increasing peak flow, and decreasing the dyspnea index, which may decrease the need for mechanical ventilation.41 In another small series, a 60 : 40 heliox mixture administered to 7 intubated patients resulted in a 15% to 50% reduction in peak inspiratory pressure and a 30% to 60% reduction in PaCO2.42 A recent literature review on the use of heliox in patients of all ages with acute asthma concluded that it may be useful in the short-term management of these patients, but any clinical advantage attributable to its use seems to diminish over time.43 There is little evidence available on the use of heliox in critically ill patients with bronchiolitis. This issue was prospectively investigated in a nonrandomized study of 38 nonintubated infants with RSV bronchiolitis admitted to an ICU.44 The investigators were able to demonstrate favorable changes in respiratory status through the first 4 hours of heliox administration and a significant decrease in ICU length of stay among infants who received heliox therapy.44 In a small randomized crossover study of RSV-positive, nonintubated patients, clinical indicators of respiratory status improved during heliox administration, particularly among children with more severe disease.45 However, many of the patients required another form of respiratory support, and the study was not designed to evaluate longer-term outcomes such as ICU length of stay.45
The application of noninvasive forms of mechanical support such as continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) using either a nasal interface or full face mask has potential advantage in the patient with adequate respiratory drive. Careful titration of applied CPAP (or positive end-expiratory pressure [PEEP]) noninvasively may prevent premature airway closure during expiration and decrease gas trapping (see later discussion). The patient who develops high levels of intrinsic PEEP due to hyperinflation manifests an increased work of breathing and, ultimately, respiratory muscle fatigue, which may precipitate dramatic and rapid clinical deterioration. Noninvasive respiratory support may allow unloading of the muscles of respiration without adding to airway reactivity and has been used with success in managing asthma as well as bronchiolitis.46–48
In the patient with respiratory failure for whom noninvasive mechanical support is not feasible, intubation and mechanical ventilation is warranted. As tracheal intubation is performed in the patient with airways disease, the clinician should be watchful for complications arising from the transition to positive-pressure ventilation. In the spontaneously breathing child with severe airway obstruction, profoundly negative intrathoracic pressures develop in order to generate lung inflation. These conditions produce maximal venous return as right atrial pressure remains subatmospheric.49 The transition to positive-pressure ventilation in this setting increases juxtacardiac pressures and right ventricular afterload, resulting in decreased venous return, decreased left ventricular compliance, and decreased left ventricular end diastolic volume,49 with risk of hypotension and cardiac arrest.3
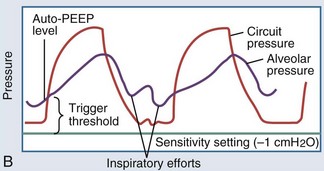
In intubated patients with status asthmaticus or bronchiolitis, low elastic recoil and increased airway resistance due to bronchoconstriction, airway edema, and mucus plugging contribute to regional gas trapping and dynamic hyperinflation (Figure 72-2, A). Gas trapping can also be exacerbated by the patient’s forced expiratory efforts, during which increased abdominal pressure is transmitted to the pleural space, potentiating premature airway closure and the development of excess or intrinsic PEEP (“auto-PEEP”). The magnitude of the auto-PEEP reflects the degree of dynamic hyperinflation in patients with severe asthma.50 Dynamic hyperinflation and auto-PEEP have an adaptive purpose in increasing the elastic recoil pressure of the lung to a level that would eventually allow complete evacuation of inhaled volume.50 However, this increase in lung volume takes place at the expense of an unfavorable change in pulmonary compliance. Other potential consequences of dynamic hyperinflation and auto-PEEP include air leak, hemodynamic compromise from sustained elevations in pulmonary vascular resistance, and increased inspiratory workload from the patient’s attempts to drop the ventilator circuit pressure below the total PEEP level (applied or set PEEP plus auto-PEEP) to trigger a breath (see Figure 72-2, B). The development of gas trapping and auto-PEEP can be inferred if the flow-versus-time waveform on the ventilator console shows initiation of inspiratory flow before the expiratory flow from the preceding breath reaches zero. Alternatively, the ventilator can quantify auto-PEEP by allowing the alveolar pressure to equilibrate with pressure at the airway opening during an end-expiratory hold maneuver. The accuracy and reliability of each of these techniques rest on the premise that all lung units communicate with the airway opening, which may not be true if bronchial obstruction is severe.51
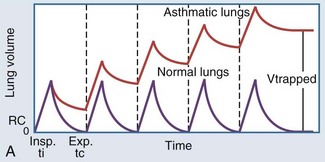
Figure 72-2 A, Dynamic hyperinflation.
Expiratory flow limitation in the asthmatic lung (upper tracing) causes incomplete evacuation of lung volume at end exhalation. Repetitive cycles of gas trapping lead to excess pressure accumulation at end exhalation (“auto-PEEP”), with a progressive shift toward ventilation on the less compliant (upper and outer) portion of the pressure-volume curve (see also Figure 35-3).
(Adapted from Stather DR, Stewart TE. Clinical review: mechanical ventilation in severe asthma. Crit Care 2005;9:581-7.)
In summary, initial ventilator settings in patients with lower-airway disease should be guided by observation, auscultation, careful ventilator waveform analysis, and attention to inspiratory plateau pressure. Ultimately, the choice of ventilator mode is not as important as a thorough understanding of how any mode might be strategically manipulated to alleviate the pathophysiology of gas trapping and auto-PEEP. It is generally preferable to allow the patient to breathe in a spontaneous ventilator mode, using a strategy of permissive hypercapnia. In spontaneously breathing mechanically ventilated patients, applied PEEP can be titrated cautiously upward as needed to improve respiratory mechanics to a level not exceeding 80% of the auto-PEEP, or until the plateau pressure begins to exceed a tolerable limit, which is usually around 30 cm H2O.51,52 If controlled ventilation is necessary, it is preferable to apply the lowest minute ventilation that provides adequate gas exchange.53 The use of neuromuscular blocking agents should be limited to the shortest feasible course because of their potentially detrimental effect on the relationship between ventilation and perfusion, and because of the risk of myopathy when these agents are administered together with corticosteroids.54 High-frequency oscillatory ventilation (see later discussion) has been used to rescue a limited number of pediatric patients with asthma and bronchiolitis who demonstrate respiratory failure refractory to management with conventional ventilation.55 One recent report recommends the use of high distending pressures to decrease airway resistance, as well as low frequencies, longer expiratory times, and muscle relaxation to minimize gas trapping.56
Sedation is an important component of managing intubated patients with lower-airway disease. Besides alleviating distress and promoting synchrony with the ventilator, sedative agents can be helpful adjuncts in limiting carbon dioxide production and reducing mechanical ventilatory requirements.51 Ketamine, a dissociative anesthetic with sympathomimetic and bronchodilatory properties, is often used for sedation in the intubated asthmatic child.57 Because of its favorable effects on airway reactivity, the inhalational anesthetic, isoflurane, may be a useful adjunct to managing severe status asthmaticus in the intubated child who is difficult to sedate or unresponsive to other therapies. The mechanism underlying its bronchodilatory properties is not well understood.58 Although isoflurane has a better safety profile than halothane when used for this purpose, periodic monitoring of renal function may be advisable in the child who requires prolonged therapy with this agent.58
 Diseases of the Alveoli
Diseases of the Alveoli
Viral Pneumonia
Defined as acute respiratory symptoms accompanied by parenchymal infiltrates on chest x-ray, pneumonia is a common syndrome in children and is most commonly caused by viral or bacterial pathogens.59 Important viral pathogens responsible for pneumonia in infants and children include RSV, influenza, parainfluenza, and adenovirus. As previously discussed, each of these is agents is also capable of producing the clinical syndrome of bronchiolitis in infants and children. The precise infectious etiology for pediatric viral pneumonias may be suggested by the physical examination, the age of the patient, and seasonal incidence patterns. Confirmatory testing through microbiologic analysis is generally sought to facilitate therapeutic decision making and cohorting of similarly affected patients. RSV is the most common viral cause of lower respiratory infection in infancy60 and primarily infects the small airways. Influenza is another very important cause of pediatric pneumonia. Infection rates in healthy children are estimated at 10% to 40% each year, and approximately 1% of these children require hospitalization.60 The course of up to 25% of infected children is complicated by lower respiratory tract disease.60 Neonates and children up to 5 years of age, especially those with underlying lung disease, congenital heart disease, immunocompromise, and other chronic conditions, seem to be at special risk for influenza pneumonia.60 Neonates are at risk for especially severe influenza syndromes which may also include apnea and sepsis.60 Infants and children older than 6 months of age, especially those in high-risk categories, are candidates for annual vaccination against influenza.61 Antiviral therapy for A and B strains of influenza are now available and can be considered for patients of appropriate age who are at high risk of complicated or severe disease.60 When administered within 48 hours of disease onset, amantadine, which is approved for use in children older than 1 year of age, may decrease the severity of influenza A disease, but data in young patients are limited.60 Oseltamivir, a neuraminidase inhibitor active against both A and B strains of influenza, has been demonstrated to decrease symptom duration when administered early in disease. When originally licensed for pediatric administration, oseltamivir was not approved for use in infants younger than 1 year.62 However, increased experience using oseltamivir in smaller infants during the 2009 H1N1 influenza pandemic produced some consensus on appropriate dosing guidelines in this age group.63 Unlike RSV, influenza is commonly associated with secondary bacterial pneumonia that is typically caused by Streptococcus pneumoniae or Staphylococcus aureus, making it especially important to consider appropriate empirical antimicrobial therapy when clinically appropriate.64,65 Parainfluenza viruses are also responsible for causing pneumonia in children, and seasonal epidemics commonly occur in autumn.60 Primary infection tends to occur in young children 2 to 6 years of age, and recurrent infection is generally less severe, except perhaps in the immunocompromised host.60 Finally, adenoviruses have been reported to cause up to 20% of pneumonias in children younger than 5 years of age, and the mortality rate attributable to the disease in this population has been reported as high as 20%.66 In neonates, adenovirus can produce an especially severe syndrome of disseminated disease and sepsis, which can present in the first 10 days of life.66 The incubation period is generally 2 to 14 days,60 and the virus can produce a profound and destructive lower-respiratory process. Necrotizing bronchitis, purulent exudative alveolitis, and hyaline membrane formation have been identified on autopsy specimens of affected patients.66 Survivors of severe adenoviral infections commonly demonstrate chronic sequelae such as recurrent wheezing and bronchiolitis obliterans.66
Bacterial Pneumonia
Most commonly, bacterial presence is established in the lower respiratory tract as a result of oropharyngeal overgrowth of environmentally acquired pathogens and subsequent introduction of these secretions into the lower airways. Children with aspiration syndromes, immunodeficiencies, and malformations of the respiratory tract are at increased risk of bacterial lower respiratory infection.67 Bacterial pathogens remain an important cause of potentially lethal pediatric pneumonias in the developing world, and they are the most important cause of severe pneumonia in Europe and North America, especially when complicated by parenchymal necrosis and/or parapneumonic effusion.59 It is challenging to establish a causal role for specific bacteria when these agents are normally found in the upper airway secretions, the specimen most commonly sampled for microbiologic diagnosis in children. The best data regarding the etiology of community acquired pneumonia come from lung-puncture studies revealing that S. pneumoniae, Hemophilus influenzae, and S. aureus are among the most important causes.59 Since the introduction of a conjugate vaccine against H. influenzae type B (Hib) in 1988, the incidence of invasive disease in infants and young children attributable to this organism has declined by 99%.60 Other serotypes of the organism, including nonencapsulated strains, may also cause pneumonia in children.60
A comprehensive review of necrotizing pneumonia cases occurring in predominantly immunocompetent children admitted to Children’s Hospital Boston between 1990 and 2005 indicates that parenchymal necrosis appears to be an increasingly common complication of pediatric bacterial pneumonia.68 In this series, S. pneumoniae was the predominant inciting organism, accounting for 22% of cases. Since 2002, many more organisms, including methicillin-sensitive S. aureus, methicillin-resistant S. aureus, Fusobacterium species, Pseudomonas species, and other Streptococcus species, have emerged as important causes of necrotizing pneumonia as well. Despite the short-term morbidity in these children, conservative management (consisting mainly of antibiotics and chest drainage) appeared sufficient to produce resolution of clinical symptoms within 2 months of hospital discharge, and marked improvement of imaging findings within 6 months.
Recent studies on the epidemiology of pediatric pneumonia complicated by parapneumonic effusion indicate that the incidence of empyema appears to have risen during the 1990s.69–71 During that period, S. pneumoniae was isolated most commonly from patients with empyema, followed by Streptococcus pyogenes and S. aureus.70,71 As in the case of necrotizing pneumonia, temporal trends in the epidemiology of pediatric empyema in the United States show a shift in causative organisms after the year 2000, when the heptavalent pneumococcal conjugate vaccine (PCV) was licensed for widespread use. A large case series reported from Texas Children’s Hospital indicates that since 2000, S. aureus has overtaken S. pneumoniae as the most common bacterial pathogen isolated from children with empyema, and the majority of S. aureus isolates in this cohort were methicillin resistant.69 In addition, nonvaccine serotypes (particularly serotypes 1, 3, and 19A) predominate among causes of pneumococcal empyema in the post-PCV era.70,72 The overall impact of widespread vaccination with PCV on the incidence of pediatric empyema across the United States is less clear. In Utah, where pneumococcal serotype 1 has always been prevalent, the incidence of pediatric empyema is still rising, while data from Texas Children’s Hospital show a decrease in the incidence of empyema since the vaccine became available.69,70
In neonates and young infants up to about 3 months of age, group B Streptococcus (GBS), Listeria monocytogenes, and gram-negative enteric organisms are the major causes of pneumonia and sepsis.60,67 Widespread maternal intrapartum antibiotic prophylaxis has influenced the incidence of perinatal GBS infection as well as its antimicrobial resistance patterns.73 The incidence of GBS sepsis has declined among very low-birth-weight infants in the era of ampicillin prophylaxis, while the incidence of Escherichia coli sepsis (largely resistant to ampicillin) has increased in the same time period.73 Perinatally acquired Chlamydia trachomatis is another important cause of lower respiratory tract infection in infants up to 12 weeks of age.67 Although uncommon, periodic epidemics of infection with Bordetella pertussis occur among incompletely immunized infants and children.67 Apnea and intermittent cyanosis progressing to respiratory failure and shock can develop in young infants infected with B. pertussis, and clinicians should have a relatively low threshold for admitting these patients to the ICU.
Therapy
In the clinical setting, one is often faced with having to select empirical antimicrobial therapy before arriving at a definitive viral or bacterial diagnosis. The presence of a focal alveolar process on chest radiographs, especially if accompanied by significant parapneumonic effusion, evidence of parenchymal necrosis, and/or abnormal peripheral blood counts and C-reactive protein, all add considerably to the predictive value for the presence of bacterial disease.59 Before demonstrating evidence of localized infection, neonates and young infants may demonstrate nonspecific but potentially ominous signs of lethargy, hypothermia, and apnea. Infants younger than 3 months of age should be treated with both ampicillin and gentamicin, and consideration should be given to adding a third-generation cephalosporin in severe cases.59 Investigation and empirical coverage for infection with B. pertussis should also be considered in infants with severe respiratory disease that features profound peripheral lymphocytosis, paroxysmal cough, and/or apnea.
For the critically ill child with community-acquired bacterial pneumonia, reasonable coverage may be assured with a third-generation cephalosporin,59,67 although some centers advocate the use of clindamycin as a second empirical agent. A macrolide antibiotic can be added in cases where infection with atypical agents such as Mycoplasma pneumoniae and Chlamydia pneumoniae is possible, particularly in patients with sickle cell disease.59,74 Although emerging resistance to penicillins in S. pneumoniae is widely recognized, high doses of cephalosporins are still appropriate in the majority of penicillin-nonsusceptible strains, so long as concurrent meningitis is not suspected, but the addition of vancomycin may be warranted in some cases.59,75 If infection with S. aureus is possible, an antistaphylococcal penicillin such as oxacillin should be added unless local resistance patterns warrant the use of vancomycin.59 In patients at risk for aspiration pneumonia and in immunocompromised children, special consideration should be given to administration of two antibiotics effective against gram-negative organisms (such as Pseudomonas) and to optimizing coverage for anaerobic organisms.
Management of pleural effusion is another important consideration in the care of the patient with bacterial pneumonia. Although drainage of parapneumonic effusions is indicated under certain circumstances, satisfactory recovery may occur in many cases without intervention.76 Recently an evidence-based clinical practice guideline was developed for the medical and surgical treatment of parapneumonic effusions in adults.77 The panel issued management suggestions according to the underlying risk of poor clinical outcome, based on effusion size and loculation as well as chemical and microbiologic analysis of the pleural fluid.77 Pleural fluid drainage was recommended for large effusions occupying more than 50% of the hemithorax, whether or not loculation or pleural thickening is present. Drainage was also recommended for purulent effusions, those with positive culture or Gram stain, or those with pH less than 7.20 as measured by a blood gas analyzer.77 In situations where drainage is indicated, more complex or invasive options such as thoracoscopic or “open” procedures are likely to be necessary for sufficient control of the effusion.77 It must be emphasized that the consensus panel’s recommendations are based primarily on case series, historical controls, and expert opinion.77
The literature on parapneumonic effusion in children also does not presently provide robust evidence on which to base clinical intervention. The effect of image-guided needle aspiration versus percutaneous pigtail catheter drainage was examined in a 5-year retrospective study of pediatric parapneumonic effusions.78 When comparing outcomes in the two groups, the authors found no difference in length of stay but did report a significant decrease in the need for second intervention in patients who received a chest drain.78 Other independent predictors for second intervention in their study population included loculation of pleural fluid and pH less than 7.2. A combination of low glucose and low pH in the pleural fluid specimen was especially predictive of the need for reintervention.78 The decision to perform thoracostomy drainage in pediatric patients with parapneumonic effusion may depend on the clinical context in which it occurs. In cases where significant pleural fluid organization has taken place, some favor the administration of intrapleural thrombolytics to facilitate evacuation of fluid through the chest drain.79 Studies assessing the efficacy of this practice have produced conflicting results. In one uncontrolled case series, 54 of 58 children (93%) with pneumonia complicated by empyema who received intrapleural tissue plasminogen activator (tPA) did not require additional surgical drainage.80 However a randomized controlled trial that enrolled 454 adults with empyema showed no outcome benefit attributable to the administration of intrapleural thrombolytics, compared to chest drainage and routine supportive care alone.81 In recent years, video assisted thoracoscopic surgery (VATS) has gained popularity as a way to facilitate chest drainage through inspection of the pleural space, disruption of adhesions, and placement of chest drains in strategic locations.79 To date, at least two prospective pediatric trials have failed to identify an outcome advantage attributable to VATS when compared to thrombolytic-enhanced chest drainage and routine supportive therapy for empyema.82,83
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree