132 Acute Bloodstream Infection
Acute bloodstream infection, which may be primary or secondary and community-acquired or nosocomial, is one of the most severe forms of infection. Frequently observed among immunocompromised and critically ill patients, bloodstream infection is rarely asymptomatic and may be associated with multiple organ failure.1–3
 Definitions
Definitions
The term bloodstream infection includes all forms of confirmed or unconfirmed bacteremia and fungemia. Acute bloodstream infections should be distinguished from septicemia, clinical sepsis, and sepsis, which refer to clinical syndromes. Definitions are summarized in Table 132-1.
TABLE 132-1 Definitions of Bloodstream Infection
| Type of Bloodstream Infection | Criteria |
|---|---|
| Positive blood culture | Recognized pathogens* identified from one or more blood cultures and not related to an infection at another body site |
| Laboratory-confirmed bloodstream infection | Positive blood culture with at least one of the following signs or symptoms: fever (>100.4°F [38°C]) or hypothermia (<98.6°F [37°C]); chills; low blood pressure (systolic blood pressure ≤ 90 mm Hg or a decrease > 40 mm Hg from baseline) |
| Primary | Laboratory-confirmed bloodstream infection or clinical sepsis occurring without a documented distal source of infection, including those resulting from catheter-related or catheter-associated infections |
| Secondary | Laboratory-confirmed bloodstream infection occurring in the presence of another documented site of infection |
| Catheter-associated | Primary bloodstream infection and presence of an intravascular access device |
| Catheter-related | Laboratory-confirmed bloodstream infection in a patient with an intravascular access device and at least one positive blood culture obtained from a peripheral vein, clinical manifestations of infection (fever, chills, hypotension), and no apparent source of bloodstream infection except for vascular access plus one of the following: positive semiquantitative culture (>15 CFU/catheter segment) with the same organism,103 positive quantitative culture (>103 CFU/catheter segment) with the same organism,104 simultaneous quantitative blood cultures with a ≥ 5 : 1 ratio CVC versus peripheral,105 and differential period of CVC culture versus peripheral blood culture positivity of > 2 h106 |
CFU, colony-forming unit; CVC, central venous catheter.
* One of the following: common skin contaminant (diphtheroids, Bacillus spp., Propionibacterium spp., coagulase-negative staphylococci, or micrococci) cultured from two or more blood cultures drawn on separate occasions; common skin contaminant cultured from one or more blood cultures from a patient with vascular access, and the physician institutes appropriate antimicrobial therapy; positive antigen test on blood and signs and symptoms with positive laboratory results not related to infection at another site.
 Epidemiology
Epidemiology
The epidemiology of bloodstream infection varies according to its source. Bloodstream infections represented 12% of all nosocomial infections reported in 10,038 patients from 1417 intensive care units (ICUs) in the European Prevalence of Infection in Intensive Care (EPIC) study,4 and similar data were found in other clinical studies.5 A worldwide prevalence study among 1265 ICUs (EPIC 2) reported bloodstream infections representing 15% of all healthcare-associated infections among 1265 participating ICUs from 75 countries.6 Almost half of all positive blood cultures obtained in a hospital are due to nosocomial bloodstream infections.7 Of these, most are primary and associated with central catheters.8
Most surveillance systems today such as the U.S. National Healthcare Safety Network (NHSN), the German Krankenhaus Infektions Surveillance System (KISS), or the International Nosocomial Infection Control Consortium (INICC) focus on catheter-associated, laboratory-confirmed, primary bloodstream infections, with reporting of bloodstream infections as episodes per 1000 device-days. Surveillance of clinical sepsis has been mostly abandoned because the definition of this infection leaves much room for interpretation and is resource demanding.8 The exception to this rule are studies among neonates; as blood cultures are often unreliable in this population.9 However, even among adults, clinical sepsis may represent up to two-thirds of central line–associated bloodstream infections (CLABSI), but focusing on microbiologically documented bloodstream infections on the other hand may underestimate true CLABSI rates.8
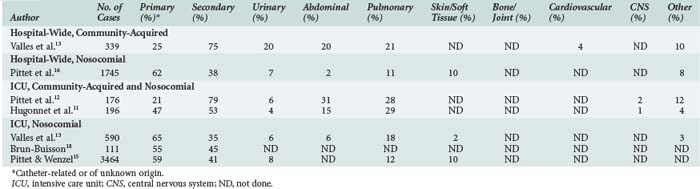
Most community-acquired bloodstream infections are secondary and due to documented infections such as pneumonia and urinary tract or soft-tissue infections (Table 132-2).10–13 Similar to nosocomial bloodstream infections, many primary bloodstream infections are associated with intravascular access devices.14–16
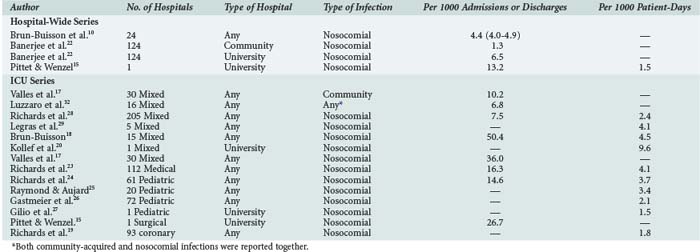
The incidence of bloodstream infection in various patient populations is presented in Table 132-3.10,13,14,17–29 The large observed differences may be related to variable definitions and reporting systems. Thus, comparisons and benchmarking should be done with caution.30,31
 Microbiology
Microbiology
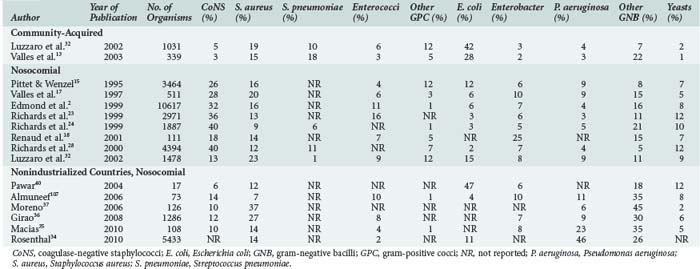
The distribution of microorganisms causing bloodstream infections varies according to source, age category (neonates, children, adults), and resources available for healthcare (Table 132-4).* In most institutions, a shift in predominant organisms from gram-negative bacilli to gram-positive cocci has been observed over the past 2 decades.11,15,28 However, in countries with limited resources, gram-negative pathogens and, among these, non-fermentative organisms such as Pseudomonas spp. and Acinetobacter spp., are still predominant.34–38 The predominance of non-fermentative organisms may be ascribed to contamination of infusates and thus to breaches in basic infection control procedures.35,42 Such breaches are likely due to the multiple use of infusates or single-use vials and a lack of respect of aseptic conditions. The shift towards gram-positive cocci seen in high-resource countries is largely due to the use of intravascular devices and the fact that the proportion of patients with risk factors such as neutropenia, solid organ and bone marrow transplantation, or the use of immunosuppressive agents has increased. The current high density of medical facilities and unrestricted access to medical care for the majority of the population in most developed countries have played major roles in the prescription of antibiotics very early in the course of most infections. In addition, the widespread use of broad-spectrum antibiotics, either for therapy or surgical prophylaxis, may be partially responsible for the increase in the relative proportions of coagulase-negative staphylococci (CoNS) and enterococci. The proportion of Candida spp., especially infections with non-albicans spp., has considerably increased in many institutions, although recent studies suggest a trend toward fewer Candida infections, at least in North America.43,44 Prolonged treatments with multiple antibiotics, the use of intravascular devices, total parenteral nutrition, and prolonged neutropenia in patients with cancer have been identified as independent risk factors in this context.45–52
CoNS are the most common pathogens isolated from blood cultures, especially in primary bloodstream infections.7 Often considered contaminants, the detection of CoNS may not always be harmless; associated mortality up to 18% has been reported.7 In contrast, mortality from Staphylococcus aureus bloodstream infection ranges between 13% and 25%, with higher rates for nosocomial than for community-acquired infection.53,54 Detection of S. aureus on catheter tips is a predictor for subsequent bacteremia, even in the absence of clinical signs and negative blood cultures at the time of catheter removal.55–57 Likewise, bloodstream infections due to Candida spp. have a poor prognosis. Mortality with this microorganism ranges between 15% and 55%, especially when antifungal treatment is delayed by 3 or more days.45,58 An important shift in the epidemiology of Candida bloodstream infections has occurred over the past decades, with decreasing infections due to Candida albicans, but increasing numbers of infections due to non-albicans isolates. In particular, fungemia due to Candida glabrata has increased.59 The emergence of this species presents clinical problems insofar as it is often resistant to fluconazole.60
 Impact
Impact
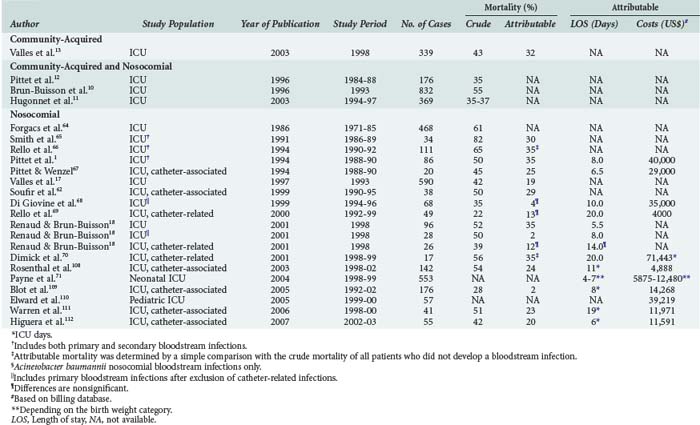
Patients with bloodstream infections are at risk for increased mortality.16,18 A meta-analysis by Siempos and colleagues found attributable mortality rates for CLABSI between 2% and 35%.61 Nosocomial bloodstream infections and, in this context CLABSI in particular, are associated with increased morbidity, prolonged length of hospital stay, and resource utilization in almost all groups of patients studied (Table 132-5).† Attributable costs and length of stay among neonates depend largely on the birthweight category, with extremely low-birthweight infants generating more expense than very low to normal-birthweight infants.71 Interestingly, mortality from secondary bloodstream infections is higher compared to primary bloodstream infections (29%–45% versus 18%–29%, respectively). Furthermore, mortality from CLABSI is lower than mortality from other primary bloodstream infections (15%–26% versus 18%–29%, respectively).16,18 Although the reason for this difference is unclear, delayed antibiotic therapy for community-acquired bloodstream infections and serious comorbidity in the context of secondary bloodstream infections may partially explain such trends.
†References 1, 10, 12–14, 17, 18, and 61–70.
Microbiological factors have been found to be important in the context of mortality among patients with nosocomial bloodstream infection, even after adjustment for major confounders intrinsic to patients’ underlying conditions.16 Pathogens that are independently associated with mortality are Candida spp. and Pseudomonas aeruginosa. CoNS are less associated with mortality compared to other pathogens, although these pathogens are isolated most frequently.16
 General Principles of Management
General Principles of Management
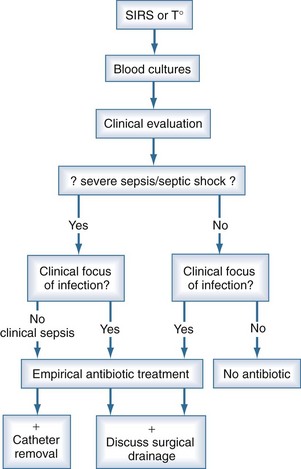
When patients are suspected to have bacteremia or fungemia, blood cultures are performed. The clinical threshold to draw blood cultures should be low, and such testing is often justified in the presence of isolated fever. This may explain why only 10% to 15% of blood cultures performed turn positive. Even in the presence of systemic inflammatory response syndrome, blood cultures are negative in 40% to 60% of cases13; however, severe sepsis and septic shock are associated with increased morbidity, mortality, and end-organ dysfunction.72 Accordingly, when sepsis is suspected, it is generally not possible to wait for results of blood cultures, and empirical antimicrobial treatment is prescribed in most cases (Figure 132-1). Owing to the low quality of blood culture sampling, the situation among neonates is even more pronounced. In one study, only 46% of blood cultures obtained from neonates contained an adequate blood volume, and only 35% were adequate submissions on the basis of collection into the correct blood culture bottle type.9 The overall positive yield of blood cultures was low, and cultures with adequate blood volume were more likely to be positive than those with inadequate blood volumes (5.3% versus 2.1%). The quality of blood culture sampling is better among older children. Of all positive cultures, 32% were contaminants, and 68% grew significant pathogens. However, only 35% of the contaminant cultures had adequate weight-adjusted blood volume, while this rate was 60% in the true bacteremia group (P < 0.001).73 Thus, inappropriate blood culture sampling is more likely to produce pseudobacteremia than correct sampling.
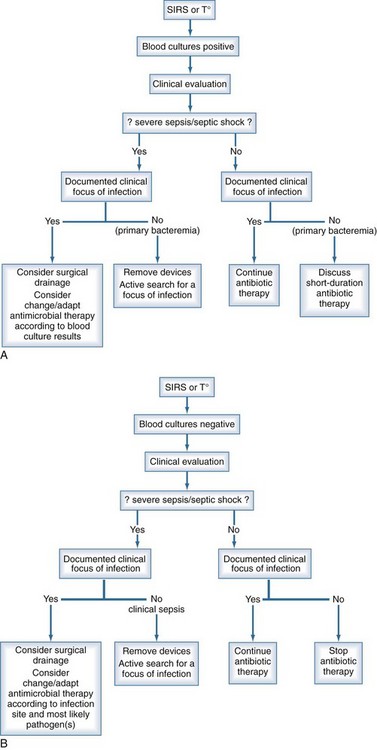
The management of bloodstream infection should combine early antimicrobial treatment and the active search for a source of infection that might require specific therapeutic measures for eradication or therapy (Figure 132-2). It has been repeatedly shown that either delayed or inappropriate antibiotic treatment is associated with higher mortality rates.11,20,74–76 Similar results were observed for candidemia, where mortality was significantly higher when antifungal therapy was delayed.58,77,78 Conversely in some studies, inappropriate antibiotic treatment was not found to be a risk factor for developing septic shock in patients with positive blood cultures,13 but the mortality of those requiring inotropic drugs was significantly higher—85% versus 75% and 58% versus 24%, respectively.
The choice of antibiotics to start empirical therapy should be based on knowledge of the local epidemiology, susceptibility of pathogens, and source of the infection. A multidisciplinary approach, including close collaboration between the physician in charge of the patient, the infectious disease specialist, and the microbiology laboratory, is of paramount importance. Such collaboration improves the accuracy of empirical therapy. Once susceptibility testing from microorganisms identified from blood cultures has been obtained, antibiotic treatment should be adjusted accordingly. In some conditions, pathogens identified from other body sites also have to be considered for treatment. In addition to antimicrobial therapy, specific measures such as drainage of abscesses, adequate surgical management of peritonitis, and removal of infected prosthetic material are necessary to control the infection. Procalcitonin-based deescalation of antibiotic therapy has been reported to reduce exposure to antibiotics by almost 30%.79–81
In the case of primary bloodstream infection or sepsis, central lines should be removed if in place at time of infection. Catheter retention may result in a several-fold increase in risk for recurrence of bloodstream infection. However, recent data suggest that antibiotic locks in addition to systemic antibiotic therapy can be used as a salvage strategy if CLABSI involves long-term catheters, signs of exit site or tunnel infection are absent, and blood cultures reveal the presence of CoNS or enterococci.82,83 Removal of the catheter is mandatory in severe or complicated infections, in the presence of shock, in case of recurrent bloodstream infection, and when microorganisms such as S. aureus, gram-negative bacilli or Candida spp. are isolated.84 Relapse, continuous fever, or bacteremia despite catheter removal requires an active search for complications such as metastatic abscess, septic thrombophlebitis, or endocarditis. Following the completion of antimicrobial therapy, careful follow-up is mandatory owing to the frequent occurrence of late complications.85,86 Recovery of S. aureus on a catheter tip may suggest the initiation of therapy even in the absence of clinical signs and negative blood cultures.55