Fig. 20.1
Acute gangrenous cholecystitis (operative photograph)
On the other hand, even before the modern era of antibiotics and other interventions, acute cholecystitis did often enough certainly resolve spontaneously. In fact, with the judicious use of antibiotics and support with intravenous fluids and analgesics, recovery from acute calculus cholecystitis without definitive intervention is expected, despite the litany of dire complications enumerated above. What happens that permits the obstructed gallbladder to recover? In many patients the obstructing stone becomes dislodged. Perhaps progressive gallbladder distension, combined with sloughing of the mucosa surrounding the stone, frees the stone to slip back harmlessly into the body of the gallbladder. Restored patency of the gallbladder outlet can be documented in many patients who recover with the help of percutaneous gallbladder decompression (cholecystostomy), and these are typically patients selected for drainage because they did not quickly resolve on their own.
The anticipated successful outcome of medically treated acute cholecystitis engendered a traditional approach of “cooling off” the inflamed gallbladder, followed by an elective cholecystectomy not sooner than 6 weeks after resolution of symptoms. This delay typically permitted a nonhostile operative field for open cholecystectomy, where acute hyperemia of inflammation had subsided, and adhesions had resolved or softened.
Diagnosis of Acute Calculus Cholecystitis
The cardinal symptom of acute cholecystitis is abdominal pain of rapid but not sudden onset, developing generally in the right upper quadrant, or else in the epigastrium. Radiation of the pain toward the right scapula tip is common. Nausea and vomiting usually accompany the pain. The attack may follow prior episodes of biliary colic, the symptoms of which are similar but self-limited. Cholecystitis symptoms persist and worsen until finally emergency medical attention is sought. On exam the patient appears worried and uncomfortable. Fever is generally absent or low-grade. Visible jaundice is rare. Abdominal exam reveals marked right upper quadrant tenderness, typically with localized guarding. A distended gallbladder or inflammatory mass can often be palpated. On the other hand, the inflamed gallbladder may be protected by the right costal margin. In this case, a deep inspiratory breath will bring the gallbladder down to the palpating fingertips. “Murphy’s sign,” in common usage, is the abrupt cessation of inspiration due to pain caused by bringing the inflamed gallbladder into contact with the abdominal wall being depressed by the examiner’s fingers. (A positive “ultrasonic Murphy’s sign” is the analogous effect caused by the ultrasound probe.)
Basic laboratory investigation may disclose at most a modest leukocytosis unless gangrene or empyema has supervened. Similarly, liver function tests are often unremarkable. Bilirubin and alkaline phosphatase are sometimes mildly elevated, indicating either a concomitant common bile duct stone or compression of the common duct by the inflammatory reaction to an impacted gallbladder stone. Likewise, aminotransferase levels are normal unless secondary inflammation of the adjacent liver causes mild elevation. A reasonable differential diagnosis includes pyogenic or amoebic liver abscess, contained perforation of a duodenal or gastric ulcer, pancreatitis, or contained perforation of the hepatic flexure of the colon. Especially in older patients, concomitant gallbladder cancer has to be considered.
Ultrasound examination of the right upper quadrant is without doubt the single most appropriate test to evaluate presumed acute calculus cholecystitis [3]. In the hands of an experienced operator and interpreter, ultrasound reliably identifies the presence of gallbladder stones; discloses the presence of a nonmobile stone impacted in the infundibulum; suggests acute inflammation by recognizing gallbladder distension, wall thickness (edema), and pericholecystic fluid; discovers additional liver pathology; measures the caliber of the intra- and extrahepatic bile ducts; assesses the status of the right kidney; and may, absent overlying bowel gas, provide a useful view of the head of the pancreas (Fig. 20.2). If ultrasound confirms calculus cholecystitis in a non-jaundiced patient, then no other test is necessary before executing the therapeutic plan.
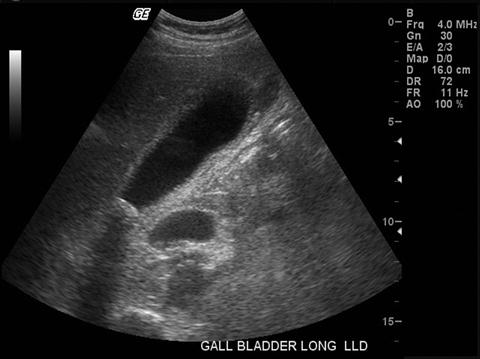

Fig. 20.2
Abdominal ultrasound examination demonstrating distended gallbladder with thickened gallbladder wall and impacted gallstone within infundibulum of gallbladder
Unfortunately, quality ultrasound studies are not available in all emergency departments, and certainly not at all hours of the day or night. Computed tomography (CT) scan is often more readily obtainable, is far less operator dependent than ultrasound, and physicians and surgeons are generally more comfortable interpreting CT scans themselves, and acting upon their findings. As a consequence, CT scanning is often performed for all acute or potentially acute abdominal conditions, including likely cholecystitis. The disadvantages of CT scans are known: radiation exposure, potential nephrotoxicity from IV contrast infusion; sequestration of the patient from caregivers for a potentially critical period of time; and expense. Additionally, CT scan is much less sensitive than ultrasound for confirming the presence of gallstones. CT scan does of course reliably confirm signs of acute cholecystitis: gallbladder distension, wall edema, pericholecystic edema, contained perforation (Fig. 20.3). Furthermore, alternative diagnoses are likely to be ruled out or in with certainty. What if CT scan confirms a strong clinical suspicion of acute cholecystitis but gallstones are not seen? While a simple ultrasound will settle the matter of stones, the therapeutic plan is nearly always unchanged by that added knowledge.
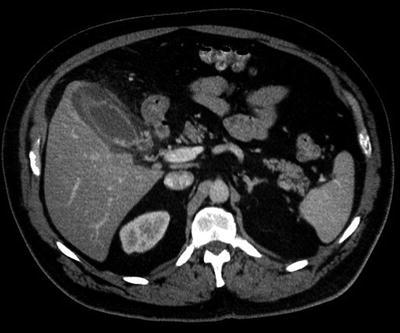

Fig. 20.3
Computed tomographic scan of abdomen showing signs of acute cholecystitis, including gallbladder wall thickening and pericholecystic edema
Treatment of Acute Calculus Cholecystitis
The medical treatment of acute cholecystitis is straightforward. The patient is admitted to the hospital and made NPO to minimize gallbladder stimulation and aggravation of nausea and vomiting. Nasogastric suction is not necessary. Intravenous hydration and parenteral pain medication are given. Antibiotics are withheld in the absence of signs of infection, but fever, leukocytosis, or other indications of sepsis are sufficient to initiate antibiotic treatment. The expected pathogens include mainly gram-negative bacilli (E. coli, Klebsiella, Enterobacter), for which the antibiotic regimen is chosen. Patients symptomatically improve because of this supportive treatment, regardless of the pathological condition of their gallbladder. They feel reassured by the attention received in the hospital, and claim to tolerate sips of clear liquids. True recovery, however, requires complete resolution of right upper quadrant tenderness, and the temptation of a flavorful fried meal should bring a guilty grin of anticipation.
In the pre-laparoscopic era, medical resolution of acute cholecystitis followed by interval open cholecystectomy was generally effective, if admittedly inefficient. But failure of medical treatment required operation—either cholecystectomy or, when that was not safe, open cholecystostomy. After several days of failed treatment, even open cholecystectomy could be a tough and even treacherous operation. Cholecystectomy earlier in the acute attack, surgeons observed, was likely to be easier and safer. Adhesions were less dense and vascular, and edema between the gallbladder wall and the liver bed facilitated dissection of this plane. A policy of “early operation” for acute cholecystitis developed, in which “early operation” meant during the index hospitalization, at the first convenient opening in the surgeon’s operating schedule. The early operation plan has been shown to be as safe as delayed cholecystectomy, while saving hospital days and preventing intercurrent biliary attacks.
Elective laparoscopic cholecystectomy rapidly replaced open elective cholecystectomy, in spite of the early frighteningly large risk of common bile duct injury. Acute cholecystitis was at first considered a contraindication to attempting laparoscopic operation. To preserve the advantages of minimally invasive cholecystectomy, many surgeons returned to the approach of medical treatment followed by delayed cholecystectomy. Fortunately the hazards and perceived difficulties of laparoscopic cholecystectomy in the face of acute inflammation have gradually been answered by experience, improved operative technique, and ongoing development of laparoscopic instrumentation. Once again, laparoscopic cholecystectomy early in the course of acute cholecystitis is demonstrably as safe as delayed operation, saves resources, and prevents further attacks [4–6]. The likelihood of needing to convert to an open operation is the same, whether done early or delayed.
The exact optimal timing of “early cholecystectomy” remains the subject of study and discussion. From the patient’s point of view, the disease and its attendant misery and inconvenience should be resolved as soon as safely possible, i.e., as soon as resuscitation is complete and medical comorbid conditions have been optimized. From the gallbladder’s perspective, so long as a stone is obstructing the infundibulum or cystic duct, the situation is getting worse. Distension, edema, hyperemia, lymphatic congestion, cystic duct lymph node enlargement are progressive. An intact gallbladder wall soon may face ischemia and necrosis. A few bacteria proliferate—will antibiotics arrive in time? and so on. A convenient window of 72 h after diagnosis and admission no longer qualifies as “early,” because on a continuum of time, the earlier the operation is undertaken, the easier and safer the operation will be, the smaller the risk of having to convert to open cholecystectomy, and the sooner the patent is likely to leave the hospital and resume normal activities [7, 8].
However early the operation is conducted, whether laparoscopic or open, it must be given its due respect as a major, potentially challenging procedure. In other words, all possible support systems need to be in place or readily available. Are the anesthesiologists and recovery room nurses prepared for a potentially sick patient? Is the scrub and circulating operating room (OR) team intimately familiar with laparoscopic equipment? Is radiology rapidly available for intraoperative cholangiography? Are surgical assistants highly qualified? And for the neophyte attending surgeon, is senior backup easily at hand? In all but the busiest medical centers, these requirements argue against conducting the operation at night. With proper personnel and dedication, a weekend cholecystectomy seems reasonable. The surgeon should be relaxed and rested, not overly stressed or sleep deprived, in order to perform the operation with the requisite equanimity. An “acute care surgery” practice model can provide the apparent paradox of an earlier operation, still performed during regular daytime hours, with less disruption of the elective surgical schedule [9]. On the other hand, the patient will be ill-served by a surgeon who lacks an elective gallbladder practice and an interest in biliary disease. In other words, we agree with Strasburg that the operation for acute cholecystitis demands a surgeon who demonstrates expertise in complicated laparoscopic cholecystectomy [10].
Operative Technique in Detail
Laparoscopic Cholecystectomy
The operation for acute cholecystitis is merely an adaptation or modification as necessary of the standard laparoscopic cholecystectomy, with which the surgeon must be entirely familiar. What follows is a description of the technique as it has evolved in our clinic.
The patient is positioned supine on the operating table with arms extended or tucked according to surgeon and anesthesiologist agreement. The position must accommodate the possibility of maneuvering a C-arm into position for a cholangiogram, as well as attaching a self-retaining retractor (e.g., Bookwalter or Omni) in the event of an open operation. A Foley catheter is used, anticipating a lengthy operation. Antibiotic prophylaxis is used unless therapeutic antibiotic coverage is already ongoing. Deep venous thrombosis prophylaxis is instituted as well.
A standard four-port approach is used, beginning with an umbilical Hassan cannula placed using an open sharp technique. Insufflation to about 15 mmHg (adjusted higher or lower according to circumstance) allows inspection using a 10 mm 30 laparoscope. The lateral 5 mm port is placed near the anterior axillary line nearly opposite the umbilicus (higher if the patient is very tall). The patient is placed in reverse-Trendelenburg, left side down position, to allow omentum and colon to fall away from the gallbladder. Next an 11 mm epigastric port is inserted, entering the abdomen to the right of the falciform ligament (sometimes made easier by pulling the ligament to the left). The fourth port is not introduced until the need for gallbladder decompression has been determined. To do this, adhesions are separated from the gallbladder fundus. Omentum is pulled away with a blunt grasper. Then, exploiting the plane along the gallbladder wall, a Maryland-type dissector or, even better, the blunt-tipped suction-irrigator is insinuated and stroked upward and downward to gently separate tissues. Usually a turgid phlegmon of omentum, mesocolon, and colonic wall can be peeled off from the gallbladder in one piece, revealing the diseased organ. At least, a space must be cleared on the dome, to permit decompression as distension will otherwise preclude grasping the fundus. Once the need for decompression is determined, the fourth trocar is placed so as to provide a direct pathway through the dome along the long axis of the gallbladder, which can be partially stabilized using an instrument inserted through the epigastric port. The distended viscus may be aspirated using a long needle and syringe; a specimen is easily gotten for culture, but emptying the gallbladder is tedious and incomplete. Our preferred option is to reintroduce the stylette into the fourth port, and thrust this port directly into the gallbladder, carefully avoiding a through-and-through injury. Once the trocar is inside, the stylette is removed and suction applied to rapidly empty the fluid contents. A Lukens trap in the suction circuit may be used to secure a sample for culture. Some spillage of content is inevitable as the trocar is retracted. A grasper through the lateral port seizes the fundus, ideally closing the perforation as well, though closure is inessential, and regrasping elsewhere to provide optimal retraction and exposure of dissection is far more important. Upward retraction of the fundus now facilitates separation of the remaining adhesions and exposure of the infundibulum. The relationship of the pylorus and duodenum are assessed; a fistula to the duodenum may be encountered and must be recognized and repaired.
The next step is to grasp the infundibulum, where all too often an impacted stone renders this plan impossible. If the stone can be milked back into the gallbladder, the problem is solved. Otherwise, grasp the body of the organ above the stone; to attempt to grasp below it is unsafe until dissection confirms the safety of the common duct. Open the peritoneum over the leading edge of the gallbladder where its junction with the cystic duct is suspected. With the infundibulum pushed medially, continue in the subperitoneal plane posteriorly first, staying well up on the gallbladder wall, proceeding as far toward the fundus as possible. This opening is then deepened, gently spreading with the dissector and taking small bites of edematous connective tissue with the hook cautery. This is the first step in achieving a “critical view of safety” [11]. The dissection is carried anteriorly on the gallbladder, skirting above the enlarged cystic duct lymph node, and superficial to the hidden cystic artery. Again, peritoneum and subjacent connective tissue are divided as far toward the fundus as practical without frequent changes of grasping position.
The cystic duct and artery are commonly inapparent, concealed by edema and an enlarged cystic duct node. As gently as possible, peritoneum, fat and connective tissue is teased and swept away from the front and back of the gallbladder hilum; forceful grasping or cautery of tissue is avoided until duct and artery are brought into view. Often, the critical maneuver is the mobilization of the enlarged and inflamed cystic duct node. Having opened the peritoneum above the node, it is dissected from the gallbladder surface and gradually rolled downwards in the direction of the hepatoduodenal ligament. In this way, small bleeders can be cauterized with less risk, and dissection a plane too deep is more likely to open the gallbladder than the common duct. The field is hyperemic; some oozing is inevitable. The node can often be teased downward with the tip of the suction-irrigator, simultaneously maintaining adequate visualization with a combination of suction and hydro-dissection. Usually the discrete vascular pedicle of the node will be identified and, once dissected, can be cauterized and divided. The node may be excised and removed, or merely swept further downward.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







