84 Acquired and Congenital Heart Disease in Children
 Physiology
Physiology
Circulatory Changes at Birth
During the transition from intrauterine to extrauterine life, major circulatory changes occur which have important implications for the clinical care of the newborn.1,2 At birth in the normal newborn, the low-resistance placenta is eliminated from the circulation, resulting in an immediate increase in systemic vascular resistance (SVR). The pulmonary vascular resistance (PVR) falls when the lungs become responsible for gas exchange, and the fetal channels, foramen ovale, and arterial duct become redundant and close. In addition to altered hemodynamics in babies born with congenital heart disease, some babies with structurally normal hearts have a persistent right-to-left shunt after birth due to failure of the transition from fetal to postnatal circulation. Babies with this circulatory pattern, which is characterized by failure of the PVR to fall, have persistent pulmonary hypertension of the newborn (PPHN).3 PPHN is one of the two principal causes of nonpulmonary cyanosis in the neonate, the other being cyanotic congenital heart disease.
Physiology of the Neonatal Myocardium
The neonatal myocardium is functionally immature.4 Age-dependent changes in intrinsic function and integration with a maturing circulation determines its response to insults such as hypoxia and ischemia.5
Healthy infants have higher plasma concentrations of catecholamines and higher-density cardiac sympathetic innervation than older children and adults. This may partly explain the reduced ability of neonates to increase cardiac output in response to endogenous or exogenous catecholamines. Children in heart failure also have higher plasma catecholamine concentrations6 but reduced densities of β-adrenergic receptors compared to age-matched controls.7 The effects of this are similar to those seen with exogenous agonist-induced desensitization. Children with severe heart failure show evidence of uncoupling of β1-adrenergic receptors from the enzyme adenylcyclase7 and other maladaptive responses that result in reduced response to receptor agonists. In addition to heart failure, chronic hypoxia, such as is seen in cyanotic congenital heart disease, induces activation of the sympathetic nervous system, with resultant adrenergic receptor desensitization. Developmental aspects of myocardial support have recently been reviewed.8 The characteristics of the neonatal ventricle are listed in Table 84-1.
TABLE84-1 Characteristics of the Neonatal Ventricle
| Comparison to Mature Ventricle | |
|---|---|
| Contractility | Contractility of the neonatal ventricle is reduced compared to the mature ventricle. |
| Compliance | Neonatal ventricle inherently noncompliant compared to mature ventricle |
| Augmentation cardiac output | Little stoke volume reserve due to low compliance. Therefore cardiac output is highly heart-rate dependent in neonates. |
| Afterload | Neonatal ventricle tolerates increased afterload poorly. |
| Energy substrate | Lactate is primary substrate of neonatal ventricle under aerobic conditions. Glucose metabolized under anaerobic conditions. By 1-2 years, changes over to primary adult substrate, free fatty acids. |
Congestive Heart Failure
Although the basic pathophysiologic mechanisms of heart failure have age-independent common mechanisms, the presentation and management of heart failure changes with age. The overwhelming cause of heart failure in the first year of life is congenital heart disease, usually with an intracardiac left-to-right shunt or a ventricular obstructive lesion (Table 84-2). By contrast, the primary abnormality in adult heart failure is usually left ventricular dysfunction. Heart failure in adults is often gradual in onset; the neonate has little functional reserve, resulting in rapid decompensation and an emergent presentation.
TABLE84-2 Common Causes of Heart Failure in Childhood
| Neonate < 2 Weeks Age | Neonate > 2 Weeks Age, Infant | Older Child |
|---|---|---|
| Congenital Heart Disease | ||
| left-sided obstructive Lesions | left-to-right shunt lesions | any lesion |
| Other Causes | acquired heart disease | |
| arrhythmias | ||
| • Incessant supraventricular tachycardia | ||
| congenital myocarditis | ||
| severe ventricular dysfunction | ||
| • Due to birth asphyxia, sepsis, or severe metabolic disorders | ||
The clinical features9 of heart failure in infants are listed in Table 84-3. A prominent sign of cardiac failure in infancy is difficulty in feeding secondary to increased respiratory rate and effort. This equates to exertional dyspnea in the older child or adult. Failure to thrive results and leads to the classic “wizened” appearance. Although hepatomegaly is a common sign of heart failure in infants (resulting from an increase in total circulating volume and hepatic venous congestion), peripheral edema, ascites, and pericardial or pleural effusions are much less commonly seen than in adults. One relatively common feature of severe heart failure in infancy is the occurrence of compression of the bronchial tree—particularly the left mainstem or lower lobe bronchus—secondary to extrinsic compression by an enlarged left atrium or pulmonary artery. This can cause airway obstruction and associated lobar collapse, or localized hyperinflation due to distal air-trapping. Long-standing extrinsic compression may rarely cause tracheobronchomalacia, resulting in long-term respiratory difficulties even after resolution of heart failure.
TABLE84-3 Clinical Features of Heart Failure in Infants
| Respiratory Signs |
| Other Signs |
Cyanosis
Cyanosis is the visible manifestation of greater than 5 g/dL of reduced deoxygenated hemoglobin in cutaneous blood vessels, and is a prominent feature in many types of congenital heart disease. Peripheral cyanosis results from high oxygen extraction ratios across the tissue vascular bed, reflecting low tissue blood flow or high tissue oxygen demand. Central cyanosis results from desaturation of arterial blood, which may be due to pulmonary disease or right-to-left shunting of deoxygenated systemic venous blood in association with a congenital heart defect. Pulmonary and cardiac causes of central cyanosis can usually be differentiated by allowing the child to breathe 100% oxygen (a “hyperoxic test”), which will result in a substantial improvement in oxygen saturation the case of cyanosis of pulmonary origin but have little effect on the child with cyanosis due to right-to-left shunt.10 During administration of 100% oxygen, arterial oxygen tensions (PaO2) above 160 mm Hg are highly suggestive of a noncardiac diagnosis, and a PaO2 over 250 mm Hg excludes it. Occasionally, differential cyanosis is seen where one or both of the upper limbs are normally saturated and the lower limbs cyanosed. The cause is deoxygenated blood traversing the arterial duct to enter the aorta distal to the origin of one or both subclavian arteries and supplying the lower limbs, while oxygenated blood from the left ventricle predominantly supplies the upper limbs.
Pulmonary Vasculature and Pulmonary Hypertension
The pulmonary vascular bed is of central importance to the manifestations of congenital heart disease from the first hours of life. Pulmonary vascular resistance usually falls dramatically in response to aeration of the lungs with the first breaths. Thereafter, the smooth muscle of the pulmonary vascular bed thins gradually during the first months of life, with associated fall in PVR to adult values by approximately 2 months of age. In infants with congenital heart lesions where an intracardiac communication between the systemic and pulmonary circulations is present, such as a ventricular septal defect (VSD), the fall in PVR encourages flow into the low-resistance pulmonary vascular bed, and a left-to-right shunt develops. In response to the increased flow and subsequent shear stress this induces, progressive structural changes occur in the pulmonary arteries and arterioles. Initially these changes consist of accelerated extension of muscle to the distal “non-muscular” pulmonary arteries and medial muscular hypertrophy in the proximal muscular arteries. Later changes involve gradual hypertrophy of the arterial intima, with deposition of collagen and elastin leading to gradual luminal obstruction and eventual occlusion. Associated with this is the development of plexiform lesions, the histologic hallmark of pulmonary vascular disease. Mild pulmonary vascular changes are of little significance to the cardiac intensivist; however, children with more extensive medial muscular hypertrophy of the pulmonary arteries are at risk of labile pulmonary hypertension (PHT) in the postoperative period (see later). The extent of pulmonary hypertensive changes frequently determine the feasibility of surgical options. Children with established fixed high PVR are unsuitable for corrective surgery, as surgical separation of the two circulations in the face of fixed high PVR will result in immediate right ventricular failure. Smaller elevations in PVR determine operability in the single-ventricle Fontan circulation (discussed later). Calculation of PVR and the response to varying vasodilators can be achieved following a pulmonary reversibility study in the cardiac catheter laboratory.11–13
 Circulatory Support in Children
Circulatory Support in Children
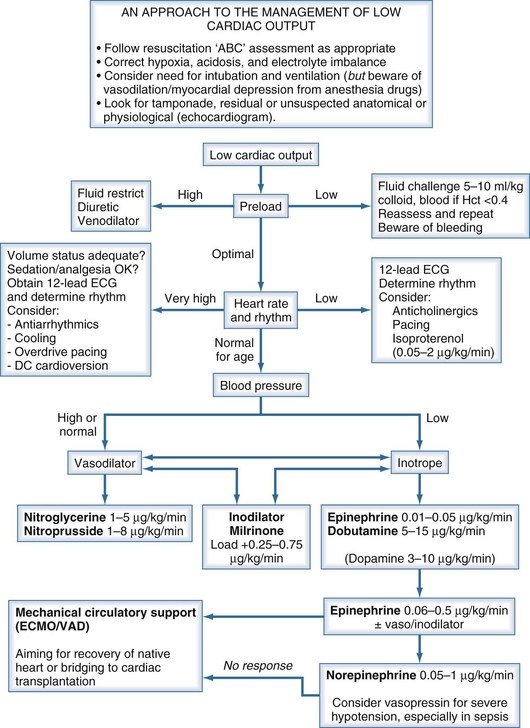
Children presenting with circulatory failure14 must initially be assessed and managed according to standard resuscitation algorithms. These require that adequate oxygenation and circulating volume be achieved. If cardiac output remains low, cardiovascular drug therapy is usually indicated. The developmental differences previously noted serve to emphasize the need to adopt age-appropriate pharmacologic strategies when supporting the failing myocardium of the neonate and infant.15–17 If cardiac output remains low despite application of such measures, mechanical circulatory support should be considered (Figure 84-1).
Pharmacologic Support
β-Adrenergic Agonists
Clinical and experimental studies have demonstrated marked age-related differences in the hemodynamic response to inotropic therapy. Although some of the observed differences may be accounted for by differences in drug pharmacokinetics, the variable maturation of the sympathetic nervous system, its receptors, and the cardiac myocytes mitigate against the recommendation of narrow specific dose ranges for the use of catecholamines in neonates and children.8
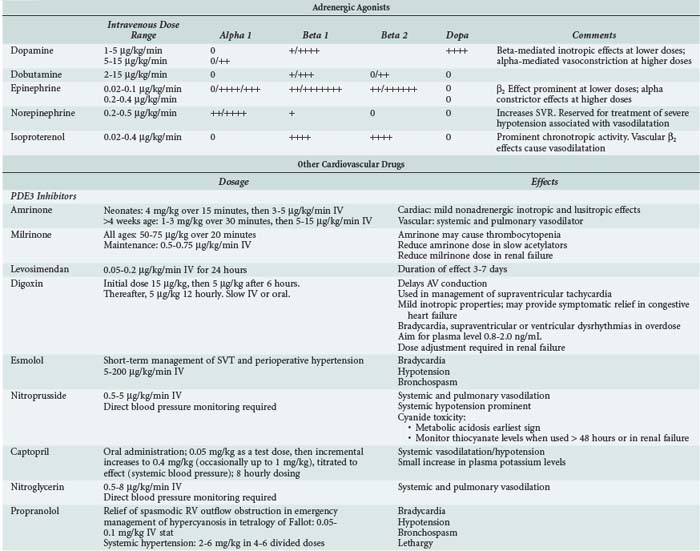
In clinical pediatric practice, adrenergic agonists are titrated to hemodynamic effect much as they are in adults (Table 84-4). When systolic ventricular function is impaired, low-dose epinephrine is commonly used as the first-line inotrope, although dobutamine and dopamine still have their advocates. Dopamine was formerly preeminent but is now less favored because of its noncardiac adverse effects.18 Additional agents should be administered according to assessment of response, judged clinically and from available hemodynamic monitoring. Higher-dose epinephrine, norepinephrine, or vasopressin can be used in refractory circulatory failure, particularly if vasodilation is present, such as occurs occasionally after cardiopulmonary bypass in children.19,20 Isoproterenol is a nonspecific β-adrenergic agonist whose principal cardiovascular effects are vasodilation and increasing heart rate. The drug is rarely used in intensive care except as a chronotropic agent where heart rate is critically low and cardiac pacing not yet established. Caution is needed when higher-dose catecholamine support is used in the neonate, as these can induce a rise in ventricular end-diastolic pressure (EDP) in a ventricle already developmentally noncompliant. Catecholamine-induced myocardial necrosis has been identified in neonatal animal models.21,22
Phosphodiesterase Inhibitors
Phosphodiesterase (PDE) inhibitors have emerged as important agents in the management of neonates and children with cardiac failure. The cardiovascular actions of the clinically available PDE3 inhibitors, amrinone,23 milrinone,24 and enoximone, are similar (Table 84-5). By inhibiting breakdown of cyclic adenosine monophosphate (cAMP), intracellular calcium accumulation is promoted and augments the contractile state of the myocyte. In addition, reuptake of calcium—a cAMP-dependent process—is also augmented, and these agents may therefore enhance diastolic relaxation, a particularly important aspect of neonatal cardiac function. In a recent multicenter randomized controlled study of neonates and young children following cardiac surgery, prophylactic administration of milrinone reduced the incidence of low cardiac output.25 Clinical studies in infants and children have demonstrated a synergistic effect when β1-agonists and PDE inhibitors such as amrinone, milrinone, or enoximone are coadministered, and this effect may be greater in neonates than in adults. In clinical use, the vasodilating action of the PDE3 inhibitors is prominent, a useful property given the usual well-documented pattern of low cardiac output associated with rising SVR and PVR in young patients following cardiac surgery.26
TABLE84-5 Strategies to Prevent and Treat Pulmonary Hypertension
| Strategy | Comment |
|---|---|
| Anatomic investigation | Rule out residual or undiagnosed anatomic abnormalities |
| Permit right-to-left decompression | Deliberate residual ASD acts as “pop-off” in at-risk situations |
| Analgesia/sedation | Facilitate ventilation; minimize sympathetic influences |
| Avoid acidosis | Respiratory and metabolic acidosis raise PVR |
| Maintain oxygenation | Normal/high alveolar and mixed venous PO2 lower PVR |
| Optimize hematocrit | Ensures optimal oxygen delivery and higher mixed venous PO2 |
| Optimize cardiac output | Ensures optimal oxygen delivery and higher mixed venous PO2 |
| Pulmonary vasodilators | Selectively reduce PVR |
Systemic Vasodilators
In children, sodium nitroprusside is frequently the systemic vasodilator of choice because of its powerful arteriolar dilating properties and short half-life which render it both effective and highly titratable. Nitroglycerin is an alternative short-acting drug which acts as an arteriolar dilator at higher doses but is an effective venodilator at lower doses. Phentolamine, a long-acting α-adrenergic blocker, is used in some centers for children undergoing surgery for congenital heart disease.27,28
For longer-term vasodilator therapy in children able to absorb enterally administered drugs, angiotensin-converting enzyme (ACE) inhibitors such as captopril and enalapril are used.29 They have peripheral vascular and neurohormonal effects, as well as direct effects on the myocardium through activation of intracellular signaling pathways involved in growth and apoptosis of cardiac myocytes and fibroblasts. Studies in adults have established that ACE inhibitors improve survival and symptoms in heart failure, in part because of their favorable effects on cardiac remodeling. Evidence for the use of ACE inhibitors in children is much less clear. Acute hemodynamic benefits have been demonstrated in children with heart failure due to left-to-right shunts and systolic dysfunction of the systemic ventricle. Prolonged treatment with ACE inhibitors has been shown to be effective in reducing not only LV volume overload but also LV hypertrophy in the hearts of growing children with chronic LV volume overload.30,31 The results of a randomized controlled trial of the use of ACE inhibitors in infants with single-ventricle circulations is awaited.32
Digoxin
Digoxin may have weak inotropic actions through its inhibitory effect on Na+/K+-ATPase and may also have peripheral effects that attenuate the actions of the neurohormonal system. Several adult studies have shown that digoxin improves symptoms in heart failure.33 Although no studies have shown survival improvement,33,34 there is a resurgence of interest in defining the role of digoxin in the management of heart failure. Digoxin is widely used to treat heart failure in children, although as in adults there are few data supporting or refuting its use.17
Diuretics
Standard practice is to use diuretics in virtually all children with heart failure.17 There are no pediatric studies showing that diuretic therapy reduces morbidity or mortality, but a recent adult study has shown that the diuretic, spironolactone, improves survival in adults with heart failure.35
Potent diuretics such as furosemide are widely used in heart failure treatment in childhood36; in the perioperative period, controlling fluid balance is crucial, and renal function may be impaired. The intravenous (IV) route is preferred in these situations. Studies have shown that continuous infusion leads to smoother control of fluid and electrolyte shifts than intermittent IV bolus administration.36
Beta-Blockers
Although there is increasing evidence of survival benefits accruing from beta-blocker therapy in adults with moderate and severe heart failure,37,38 evidence of similar benefits in children with heart failure is limited.29,39,40 A recent publication suggests that the benefit of adding beta blockade to ACE inhibition is minimal.41 While it might be reasonable to extrapolate adult survival advantages to older children with heart failure, extreme caution should be exercised in seeking to apply such therapy in the neonatal period.
Levosimendan
Levosimendan offers new therapeutic possibilities in the management of patients with severe ventricular dysfunction by improving cardiac contractility and vasodilatation without affecting intracellular free calcium.46 This drug enhances the sensitivity of cardiac myofilaments to calcium. The myocardial effects of levosimendan show improvement not only in systolic function but also in improved diastolic function, which is significantly impaired in severe heart failure. Anecdotes about the efficacy of levosimendan continue to be reported47 to add to the small previously published studies such as that of Namachivayam et al.48 It is, however, disappointing not to be able to report the results of more substantive pediatric trials. One of the problems with understanding the clinical utility of levosimendan has been to quantify the magnitude of its lusitropic effects, separating this from inotropic and chronotropic effects. Recently Jorgensen et al.49 published an elegant study of the use of levosimendan in a carefully monitored group of adult patients with aortic valve disease. This study demonstrated unequivocally that levosimendan exerts a direct positive lusitropic effect, shortening isovolumic relaxation time and improving LV filling.
The potential for tight control of blood glucose to improve cardiac outcomes in children has recently been highlighted.50 Further evidence from clinical trials such as the CHiP trial51 are required before tight control is routinely adopted in pediatric critical care.
Other Inotropic Agents
Triiodothyronine (T3) plays an important role in the regulation of heart metabolism,42 up-regulating β-adrenoceptors and increasing cardiac myocyte contractility.43 Clinical studies have shown that T3 supplementation can produce elevation in heart rate without concomitant decrease in systemic blood pressure44 and may enhance cardiac function reserve in infants after cardiopulmonary bypass. A recent double-blind placebo-controlled trial investigated the use of triiodothyronine supplementation in children younger than 2 years of age undergoing cardiopulmonary bypass. Although some indices of cardiac function assessed by echocardiography were judged better in the T3 group, no significant differences were found in clinical endpoints including time to extubation or intensive care unit (ICU) discharge.45
Pulmonary Vasodilators and Other Strategies to Prevent and Treat Pulmonary Hypertension11
Oxygen alone is a potent dilator of the pulmonary vascular bed, with both alveolar oxygen concentration and systemic oxygen saturation having a favorable influence. Pulmonary vascular resistance is also influenced by lung volume, being raised at both low and very high lung volumes. Avoiding atelectasis, alveolar hypoxia, and pulmonary arteriolar hypoxia are simple strategies to minimize PVR and pulmonary artery pressure. Historically, most IV drugs used to treat PHT had nonselective effects, dilating both the pulmonary and systemic vascular beds. Tolazoline, prostaglandin E1, and prostacyclin are among many agents which have been used as pulmonary vasodilators. Prostacyclin is a short-acting vasodilator which acts via increasing levels of the intracellular messenger, cAMP, which has been widely used in the treatment of primary PHT in children.52 The pulmonary effects of such nonselective agents are frequently limited by their nonspecific action leading to clinically important systemic hypotension. In contrast, nitrates, sodium nitroprusside, and indeed nitric oxide act via the activation of guanylate cyclase and hence increase cellular levels of cyclic guanosine monophosphate (cGMP) which is then inactivated by PDE5.
Elevation of PVR is seen in all children following cardiopulmonary bypass (CPB),26 with reactive postoperative pulmonary hypertensive episodes typically occurring in children following correction of left-to-right shunt lesions or in those with preoperative pulmonary venous hypertension.53 These crises are particularly associated with long CPB durations and late presentation for surgery. In the current era, early corrective surgery has dramatically reduced the numbers of infants in whom PHT is a major perioperative issue. Postoperative PHT is still seen in neonates and infants in association with lesions such as obstructed total anomalous pulmonary venous drainage, truncus arteriosus, and mitral valve replacement for congenital mitral stenosis. Children with lesser elevations in PVR may also benefit from pulmonary vasodilatation, including children with predominant RV dysfunction, for instance following cardiac transplantation54 and in Fontan circulations and relatively high PVR.55 General measures associated with the prevention and treatment of PHT should be considered before deploying specific pulmonary vasodilators (see Table 84-5). In patients at high risk of PHT following cardiac surgery, left ventricular filling can be maintained by right-to-left shunting through a small, surgically created atrial septal defect (ASD). Right-to-left shunt acts as a safety valve, and while some systemic desaturation occurs, LV filling and hence cardiac output are maintained.
Nitric oxide is an endogenous endothelial-derived vasodilator and a gas at room temperature. If added to inhaled gas mixtures in children with reactive PHT, it induces selective pulmonary vasodilation.56 It is distributed to ventilated alveoli, from where it diffuses into the adjacent pulmonary arteriolar smooth muscle. Inhaled nitric oxide (iNO) has been shown in randomized controlled trials to be effective and safe therapy in neonates with PPHN. Although the evidence for outcome benefit is limited to one randomized controlled study,57 there is a substantial body of evidence to show that iNO is effective in pediatric cardiac patients, including those with acute postoperative PHT following congenital heart surgery58,59 and following pediatric heart transplantation. Inhaled nitric oxide can also be used in preoperative assessment of patients with PHT.13,60
Other candidate selective pulmonary vasodilators undergoing investigation in children include inhaled prostacyclin61; the PDE5 inhibitor, sildenafil62–64; and bosentan, an endothelin-1 receptor blocker.65–67
Mechanical Circulatory Support
Extracorporeal membrane oxygenation (ECMO) is a mature technology which has been used to support over 27,000 neonates with respiratory failure, in whom survival rates of 70% to 80% are expected. Its use in this indication is supported by randomized controlled trials that demonstrate good short- and medium-term outcomes.68 ECMO and ventricular assist devices (VADs) have subsequently been used to provide temporary circulatory support in children with intractable circulatory failure (see Chapter 93). Indications for mechanical circulatory support include selected children with problems including severe ventricular failure, refractory arrhythmias, and cardiac arrest.69,70 The aim of mechanical circulatory support in such circumstances is to provide optimal cardiac output while resting the heart, awaiting its recovery, or to achieve survival by successful support of the child to cardiac transplantation. Single-center series71 and collaborative registry figures of ECMO72 or VAD for acute postoperative indications report similar figures for survival to hospital discharge (~40%) in children who (it is assumed) would not have survived without mechanical support. Rapid-deployment ECMO has recently been reported as an effective intervention for the management of cardiac arrest in the pediatric cardiac ICU and cardiac catheter laboratory.73 Hospital survival figures for CPR-ECMO seem encouraging,74 but long-term neurodevelopmental follow-up studies are urgently needed before such strategies can be recommended unequivocally.75,76
 Cardiomyopathies
Cardiomyopathies
The two most common causes of heart failure in children are congenital heart disease and cardiomyopathy. Cardiomyopathies are primary myocardial diseases of either known or unknown cause, characterized by left or biventricular dilatation and impaired contractility; they occur in children and adults of all ages. Additional information on cardiomyopathy in adults is provided in Chapter 83. Key aspects germane to pediatrics are provided in the following discussions.
Nugent et al. reported the incidence of pediatric cardiomyopathy in a 10-year population-based study in Australian children as 1.24 cases per 100,000 children younger than 10 years of age,77 a remarkably similar finding to a recently reported U.S. study.78 Of 314 cases of cardiomyopathy reported by Nugent et al., 184/314 (59%) were dilated cardiomyopathy, 80 (25%) hypertrophic cardiomyopathy, 8 (2.5%) restrictive cardiomyopathy, and 42 (13%) unclassified, of which 29 (69%) exhibited LV non-compaction. In this study, 20% of cardiomyopathies were classified as familial, and in 8.9%, specific mitochondrial or metabolic disease etiologically linked to cardiomyopathy were identified. Of the children in Nugent’s study who underwent myocardial biopsy, 40.3% had histologic evidence of lymphocytic myocarditis according to the Dallas criteria,79 which contrasts with an incidence of lymphocytic myocarditis in adult studies of only 10%.80
Presentation
Most children present with signs and symptoms of heart failure including dyspnea, upper abdominal discomfort, nausea, and vomiting. Abdominal symptoms are often misdiagnosed as indicative of gastroenteritis, although the astute clinician will note the absence of diarrhea. It is presumed that these abdominal symptoms result from hepatic congestion and gut edema as a result of right heart failure or ischemia (from splanchnic vasoconstriction). A history of an antecedent flulike illness is strongly suggestive of a diagnosis of myocarditis. Some children with myocarditis follow a fulminant course typified by rapid onset of cardiogenic shock.81,82
Prognosis
Recent studies have reported 5-year survival rates in childhood cardiomyopathy of between 64% and 84%, although the impact of cardiac transplantation on survival rates is not clear in all studies. In contrast to myocarditis, sudden death is uncommon in children with other forms of dilated cardiomyopathy. Children with cardiomyopathies who fail to respond to conservative treatment, and especially those with ongoing requirement for IV inotropic support, ventilatory support, or mechanical circulatory support and children with recurrent arrhythmias are candidates for early cardiac transplantation.83 Late recovery of ventricular function is, however, possible.84 The prognosis for cardiomyopathy due to myocarditis in children appears to differ from adults, with survival of up to 80% among children who reach the hospital alive.85,86 Many children who survive the acute phase go on to recover normal cardiac function—in marked contrast to adults, in whom mortality rates of 20% at 1 year increased to 56% at 5 years.80
ICU Management of Dilated Cardiomyopathy and Myocarditis
In children presenting with acute heart failure, hypotension, or cardiogenic shock, β-adrenergic agonists may improve systolic ventricular function. PDE3 inhibitors such as milrinone are of hemodynamic benefit in acute heart failure, although large trials in adult heart failure have failed to show clear benefit from chronic administration.87 While metoprolol and carvedilol may be of benefit in chronic heart failure,29,39,40 they should be avoided in hemodynamically unstable children. Nasal or mask continuous positive airway pressure (CPAP) has been shown to result in symptomatic improvement both by unloading of respiratory muscles and lowering of LV afterload as a consequence of raising intrathoracic pressure.88 Children in severe heart failure have high SVRs and no ventricular reserve. Great care is therefore needed if sedative agents are administered to facilitate tracheal intubation or ICU procedures. Agents with the least effects on the cardiovascular system should be chosen and allowance made for slow circulatory times when titrating sedative doses.
The use of mechanical circulatory support with ECMO or ventricular-assist systems can be life saving in children with myocarditis or cardiomyopathy who develop cardiogenic shock.89,90 A high proportion of children who receive mechanical support for fulminant myocarditis will recover ventricular function. Those who do not may be bridged to cardiac transplantation. Clearly, survival with a recovered native ventricle is a better outcome for a child than survival via cardiac transplantation. A multicenter series86 documented a median time to return of ventricular function of 9 days in those who survived without transplantation. The absolute time limits for recovery of native ventricular function have not been established, although pragmatic decisions on whether or not to proceed to cardiac transplantation should probably be made if cardiac recovery has not occurred after 10 to 14 days of support.91
 Congenital Heart Disease
Congenital Heart Disease
Congenital heart disease (CHD) classified as moderate or severe is detected in approximately 6/1000 live births, of whom between 2.5 and 3 will require expert cardiologic care soon after birth. The presence of extracardiac anomalies in children with CHD is associated with poorer outcomes. Syndromes associated with cardiovascular involvement are of particular significance to the pediatric intensivist who must coordinate care of the cardiac and extracardiac aspects of care.92 Trisomy 21 (Down’s syndrome) is associated with a high incidence of congenital heart disease, in particular atrioventricular septal defects. Deletion of the q11 region of chromosome 22 is associated with a spectrum of cardiac conotruncal defects (e.g., truncus arteriosus, tetralogy of Fallot) and extracardiac abnormalities.93 Of the later, thymic aplasia places infants at risk from hypocalcemia secondary to hypoparathyroidism and impaired cellular immunity.
Many classifications of congenital heart lesions have been proposed. A sequential approach to the description of cardiac anatomy is most frequently employed by pediatric cardiologists, but a broader physiologic approach is more useful to the non-specialist. It is beyond the scope of this chapter to present a detailed overview of all aspects of CHD. A brief overview is presented, focusing on common lesions and information of particular importance to intensivists. Readers are directed elsewhere for more detailed coverage of pediatric cardiology,94 pediatric cardiac surgery,95 and pediatric cardiac intensive care.96
Lesions with Predominant Left-To-Right Shunt
Ventricular septal defect is the archetypal lesion associated with left-to-right shunting of blood. VSDs may occur in isolation or in association with other cardiac anomalies. Ventricular output will follow the path of least resistance, resulting in blood shunting across the defect and into the lungs, as the PVR is lower than the SVR. The magnitude of the shunt, usually expressed as the ratio of pulmonary blood flow to systemic blood flow (Qp : Qs), depends on the size of the VSD and the level of the PVR. Small-diameter defects offer resistance at the level of the ventricular septum, limiting flow from the left to right ventricle and maintaining a pressure gradient between the two chambers. Larger-diameter defects are unrestrictive, with no pressure gradient between the two ventricles, and in this situation, flow is solely dependent on the ratio of PVR to SVR. Small, restrictive VSDs rarely result in symptoms in infancy, typically presenting when a cardiac murmur is detected as an incidental finding. Infants with larger unrestrictive VSDs gradually develop congestive cardiac failure due to the increase in pulmonary blood flow which occurs as the developmental fall in PVR falls in the first weeks of life.97 Thus the consequences of a moderate or large unrestrictive VSD are increased pulmonary blood flow (high Qp : Qs) and extra volume work demanded of the left ventricle. The volume overload of the LV results in LV enlargement and failure. If large left-to-right shunts are left untreated, PVR gradually rises. Although the initial rise is the result of pulmonary arteriolar muscular hypertrophy which is reversible, irreversible pulmonary vascular obstructive disease98 eventually ensues and may result in the onset of right-to-left shunt (Eisenmenger syndrome). For this reason, steps must be taken in all children with congenital heart lesions and raised pulmonary blood flow to correct the lesion or protect the lungs by either a corrective procedure or a palliative procedure such as pulmonary artery banding before severe pulmonary vascular changes develop. With the exception of isolated atrial septal defects, most left-to-right shunt lesions which require surgical intervention present in the first year of life, with heart failure and associated development of PHT. The principal lesions are described next.
Ventricular Septal Defect
Anatomy
Ventricular septal defects occur in any part of the interventricular septum and are classified by location.99,100
Pathophysiology
Many small VSDs close spontaneously,101 but if closure does not occur, infants with unrestrictive defects will fail to thrive and develop congestive heart failure as the PVR falls in early infancy. Untreated VSD leads to PHT and eventual progression to fixed pulmonary vascular obstructive disease. Eventually, pulmonary artery pressure and vascular resistance exceeds that of the systemic circulation, leading to shunt reversal and cyanosis (Eisenmenger syndrome). Patients with a fixed high PVR are not suitable for VSD closure, since the right ventricle will not tolerate the excessive afterload of the hypertensive pulmonary vascular bed.
VSD Closure
Most VSDs are repaired as a primary surgical procedure.102 Occasionally, pulmonary artery banding is undertaken to reduce pulmonary blood flow and protect the pulmonary vascular bed in neonates in whom primary repair is high risk. This may be the case with complex defects such as multiple defects or in very small premature infants. These conservative strategies are questioned by some surgeons.103,104 Although most VSDs are closed surgically with a sutured patch during CPB, some defects can be closed at cardiac catheterization with an occlusion device.105
Postoperative Management
Most children undergoing elective VSD closure progress rapidly to extubation. Patients with severe cardiac failure or high pulmonary artery pressures preoperatively benefit from a more cautious approach in the early postoperative period, as do those with complex associated lesions. Low cardiac output or pulmonary edema may be noted in the early postoperative period as a consequence of generalized myocardial hypocontractility or due to the presence of a residual VSD. Pulmonary hypertension is relatively rare in the current era of early primary repair of VSD. Late-presenting cases may have PHT, and life-threatening pulmonary hypertensive crises can occur in the postoperative period. Surgically placed pulmonary artery catheters greatly assist in the early detection and management of such episodes.106 Junctional ectopic tachycardia (JET)107,108 and complete heart block are generic risks of surgery in the vicinity of the ventricular septum. Compete heart block may be transient, but if AV synchrony has not returned by 7 to 10 days, a permanent pacing system is required.109
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree