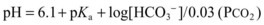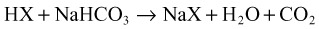160 Acid-Base Disorders
• Normal pH or serum bicarbonate values can mask an important, underlying acidosis in the setting of a mixed disorder.
• An elevated anion gap is a sign of metabolic acidosis and should be calculated on each chemistry sample.
• Arterial and venous blood gas sampling is a useful emergency department test because of the strong association between arterial and venous HCO3− and pH.
• Correlation between venous PCO2 and arterial PCO2 is lacking, although venous PCO2 levels may be used as a screening tool for hypercapnia.
• Admission lactate level and standard base excess are markers of illness severity that correlate with patient morbidity and mortality in the hospital.
• The urine ketone dipstick test is highly sensitive for serum ketosis.
• Venous and arterial lactate samples are equivalent.
• Indiscriminate use of sodium bicarbonate for the treatment of undifferentiated metabolic acidosis should be avoided.
Pathophysiology
Diagnostic Interpretation
![]() Facts and Formulas
Facts and Formulas
pH = 6.1 + Log[HCO3−]/0.03 × PCO2
Anion gap = Unmeasured anions − Unmeasured cations = Na+ − [Cl− + HCO3−]
Delta gap = Δ Anion gap − Δ HCO3 = [Calculated anion gap − 10] − [24 − Measured serum HCO3]
Calculated Sosm (mOsm/kg) = 2 (Na+) + BUN/2.8 + Glucose/18 + Ethanol/4.8
Arterial and Venous Blood Gases
The ability to substitute venous blood gas samples for arterial samples is appealing because of the pain, difficulty, and complications associated with arterial sampling. Arterial pH and venous pH vary by less 0.04 in most situations.1–3 Patients in clinical shock are an important exception, however, because arteriovenous PCO2 (and therefore pH) can vary significantly.
Despite incomplete correlation between venous and arterial PCO2, venous PCO2 may be used to screen for arterial hypercapnia. In hemodynamically normal patients, PCO2 higher than 45 mm Hg is sensitive (but less than 50% specific) for the detection of arterial hypercapnia, which is defined as PCO2 higher than 50 mm Hg. Venous blood gas screening led to a 29% reduction in arterial sampling in one study.4 Finally, arterial blood gas analysis enables precise interpretation of respiratory compensation when needed.
Standard Base Excess
SBE has been studied extensively as a resuscitation end point in trauma and as a marker of tissue acidosis. Preresuscitation base excess values are reliably linked to the degree of tissue acidosis and serve as independent predictors of mortality in critically ill patients. Base excess has been shown to correlate with hypovolemia, length of hospital stay, and transfusion requirements, whereas the rate of normalization correlates with patient survival.5,6
Lactate
Arterial lactate sampling is considered the most reliable measure for detecting hyperlactatemia; however, venous and capillary sampling is also used. Central venous sampling is highly correlated with arterial lactate measurements. Peripheral venous samples are sufficient to screen for hyperlactatemia but retain poor specificity (57%) when compared with arterial samples.7 Elevations in venous lactate should be confirmed with arterial sampling.
Anion Gap
Within serum, the requirement for electroneutrality dictates that the net serum cation charge equal the net total anion charge. The calculated difference in commonly measured serum ions is termed the anion gap (AG). It is important to note that the AG represents anions that are present but unmeasured (at least historically) and that an AG is present during health. Fortunately, the difference between unmeasured anions and unmeasured cations may change (increased or decreased AG) and therefore provide a clue to disease states (Box 160.1).
When acids are added to the system, bicarbonate is replaced by the acid anion (X) as follows:
Hyperchloremia maintains electroneutrality without altering the AG. Gastrointestinal and renal losses are the most common causes of non-AG metabolic acidosis (Box 160.2).