Fig. 40.1
Possible sites of implantation for single-kidney transplantation (KT) (top left), pediatric dual en bloc (top right), and double (two for one) KT. Normally, single and dual en bloc kidney are implanted onto the external iliac vessels. In the case of double KT, both kidneys can be implanted on the same side, on the external and the common axis. This allows sparing of the contralateral fossa, where a new kidney can be implanted, shall the first fail (used with permission of Andrea Peloso, MD)
The kidney allograft is brought into the operative field and kept cold by wrapping in iced slush. The end-to-side venous anastomosis is usually performed first under lateral clamping of the iliac vein and irrigation of the lumen with heparinized saline solution. Upon completion of this anastomosis, a small clamp can be placed on the renal vein just above the anastomosis in order to restore native venous return by removing the clamp(s) from the EIV. The arterial anastomosis is usually performed just cephalad to the venous anastomosis on the EIA using the same principles. When the kidney is from a deceased donor, a Carrel aortic patch is usually fashioned around the renal artery orifices to simplify the anastomosis. Reperfusion is performed by retrograde perfusion through the vein first followed by anterograde arterial perfusion. Once hemostasis is achieved and the optimal kidney position determined, the bladder is distended through the bladder catheter and the dome is exposed. The ureteral reconstruction is usually done using a modified Lich-Gregoir extra-vesical anterior uretero-neocystostomy. The anterolateral detrusor muscle is carefully transected to expose the underlying bladder mucosa. The ureter is trimmed to an appropriate length, the peri-ureteral tissue carefully ligated to achieve hemostasis, and the distal part spatulated. The bladder mucosa is opened sharply and a uretero-neocystostomy is performed using fine-running monofilament suture between bladder and ureteral mucosa either with or without a ureteral stent. After completion of the mucosal anastomosis, the detrusor muscle is re-approximated with absorbable or nonabsorbable monofilament suture in an interrupted fashion to loosely recreate an anti-reflux submucosal tunnel; thereby avoiding stenosis of the anastomosis the transplant procedure is completed with confirmation of the optimal position of the kidney, patency and integrity of all anastomoses, and careful wound closure in layers.
The Kuss Procedure Challenged
The extension of inclusion criteria to elderly, obese, and/or diabetic patients as well as to patients with compromised vascular and urologic conditions has been responsible for technically more demanding KT procedures.
The Compromised Vascular Patient
Nowadays, pre-transplant investigations often include duplex ultrasonography or angio-CT scan in order to delineate the status of the aorto-iliaco-femoral vessels. If severely compromised, the surgical strategy needs to be adapted by either pre-transplant or simultaneously performed endarterectomy or vessel replacement using prosthetic grafts [3, 4]. The simultaneous vascular and transplant procedures have the disadvantage of an increased risk for infection, thrombosis, bleeding, stenosis, or aneurysm formation at the site of implantation. The venous dissection is often also more difficult because of scarring and distortion of the normal anatomy. Several (small) series have reported successful outcomes with KT performed either simultaneously with or following aorto-iliaco/aorto-femoral prosthetic replacement surgery, as well as the successful implantation of the allograft renal artery on a prosthetic graft.
In the case of pre-existing vascular procedures, anticoagulation and antiplatelet aggregation therapy must be monitored carefully in order to avoid supplementary complications.
The Compromised Urologic Patient
Presently patients with untreatable lower urinary tract disease and small-volume, poorly compliant bladder with inadequate emptying do not represent an absolute contraindication to KT anymore provided that the lower urinary tract dysfunction is either adequately corrected or managed [5]. Medical therapy (using anticholinergic, alpha-adrenergic blocking drugs) and clean intermittent self-catheterization are the first-line treatments. When insufficient, surgical intervention such as augmentation cystoplasty, intermittent catheterization mechanisms (Mitrofanoff principle), or creation of a neo-bladder with bowel or ureter may become necessary. Patients with a history of recurrent urinary tract infections, vesico-ureteral reflux, and uretero-hydronephrosis can benefit from pre-transplant or simultaneous nephrectomy or nephro-ureterectomy.
When the bladder is absent or when a reconstructive procedure becomes necessary, it is preferable to reconstruct the lower urinary tract before performing the KT. Small series indicate that KT can be safely performed in patients with reconstructed bladders, as graft survival and function are comparable to patients with normal function of the lower urinary tract that have not undergone reconstructive surgery [6].
Developments During the End of the Twentieth and the Beginning of the Twenty-First Centuries
The Expansion of the Deceased Donor Organ Pool
The burgeoning crisis in organ supply challenges the transplant community to maximize and optimize the use of organs from all consented deceased donors. The persistent scarcity of deceased donor kidneys in the face of a growing wait list mandates an ongoing reappraisal of the limits of acceptability when considering whether or not to transplant a recovered kidney.
The Expanded Criteria Donor
Over the past decade, there has been an increasing shift towards increasing numbers of older donors and recipients in KT, with cerebrovascular events now being the leading cause of brain death in deceased organ donation [7]. The value of transplanting such kidneys has been questioned because of concerns over decreased graft survival, diminished longevity, and poorer predicted outcomes when compared to KT from younger donors. In October 2002, the United Network of Organ Sharing (UNOS) defined expanded criteria donation (ECD) as donor age >60 years, or age 50–59 years with at least two of the following: history of hypertension, terminal serum creatinine >1.5 mg/dl, or cerebrovascular cause of death. There is now a more and more general agreement that these kidneys should be used to improve access to transplantation for patients whose life expectancy is less than their predicted waiting time for a kidney, particularly older patients and diabetics. Despite reports of inferior graft survival and poorer intermediate-term results, Stratta et al. have contended that ECD kidneys are defined by suboptimal nephron mass, so appropriate donor and recipient selection may optimize outcomes. Rather than transplanting ECD kidneys indiscriminately, ECD kidneys should preferentially be transplanted into “low-risk” and “low-functional-need” recipients, specifically older patients with low body mass index and low immunologic risk. This strategy of profiling ECD kidneys with appropriate recipients may result in acceptable medium-term graft survival comparable to concurrently transplanted standard criteria donor (SCD) kidneys. In addition to matching strategies, ECD kidney graft survival has also improved in the most recent era secondary to advances in donor management and selection, organ preservation and assessment, recipient management, immunosuppression, and clinical experience.
The Cardiac Death Donor
Although donation rates have not changed significantly in the past decade, the percentage of transplants performed from donation after cardiac death (DCD) has increased steadily from 1.4 % in 1998 to 15.8 % in 2011 [8]. Compared with kidneys recovered from brain-dead donors (DBD), DCD kidneys are subjected to variable periods of warm ischemia after withdrawal of life support followed by declaration of death by cardiocirculatory arrest. Warm ischemia is known to be associated with acute tubular necrosis, irreversible cell damage, and reduced graft survival after KT. Despite the warm ischemia inherent with DCD recovery, numerous studies have shown comparable short- and intermediate-term graft survival rates between DBD and DCD non-ECD kidneys, although delayed graft function (DGF) is more common with DCD kidneys, with an incidence ranging from 25 to 90 %. It has been shown that older donor age and, more specifically, ECD status negatively impact on graft survival; yet, utilizing DCD-ECD kidneys as dual transplants could potentially improve outcomes. Interestingly, several studies have shown that the presence of DGF does not have the same detrimental effect on graft survival after transplantation of DCD donor kidneys as it does after transplantation of DBD donor kidneys. Two recent randomized prospective studies reported opposite conclusions regarding the efficacy of machine preservation in reducing the incidence of DGF after DCD donor kidney transplantation. However, the incidence of DGF was >50 % in both studies irrespective of the method of preservation. Early experience with extracorporeal support to reduce ischemic injury and DGF is promising, although more widespread application of this technology is limited to date.
The Dual-Kidney Transplantation
Concerns over the limited life-span of ECD or other “marginal” kidneys have resulted in an increased prevalence of simultaneous transplantation of both kidneys from a deceased donor into a single recipient. Dual-kidney transplantation (DKT) is performed to optimize outcomes by providing adequate nephron mass using kidneys thought to be unsuitable for single use, which might otherwise result in the kidneys being discarded. DKT has been shown to have comparable outcomes to single KT, including similar rates of DGF and graft survival. Criteria for allocating kidneys for DKT vary, although primary considerations include donor age, pre-existing hypertension or diabetes, donor creatinine clearance, rising serum creatinine, machine preservation characteristics, cold ischemia time, and preimplantation donor biopsy characteristics. A recent multicenter prospective analysis showed excellent short- and long-term results after allocation of kidneys from donors older than 60 years of age based on a preimplantation histologic scoring system. While DKT graft survival was similar to solitary KT graft survival in this study, it is possible that some DKTs could have had comparable results had they been transplanted as single kidneys, since the histologic scoring system allocates kidneys with only mild chronic changes to DKT. Furthermore, while donor arteriosclerosis was included in the allocation criteria, the severity and extent of these lesions were not well defined. A recent report from Kayler examined outcomes of donor kidneys with moderate (>25 %) arteriosclerosis on preimplantation biopsy when transplanted as single transplants or DKT. Single KT from donors with moderate arteriosclerosis resulted in poor 1-year graft survival (71 %) when compared to DKT (95 %), despite the use of older and more ECD kidneys in the DKT group. Appropriate recipient selection is critical to achieving good outcomes with DKT. The ideal recipient should be older than age 50 years, have adequate cardiac reserve and a low burden of iliac atherosclerosis, weight less than 80–90 kg with a BMI <30 kg/m2, and have adequate bladder capacitance. Reserving DKT for smaller individuals permits easier implantation of a technically more challenging procedure and favors functional outcomes by matching nephron mass with lower muscle mass and decreased metabolic demand. From a technical standpoint, DKT has evolved from a bilateral iliac fossa approach (either intra- or extra-peritoneal) to an extra-peritoneal unilateral approach (Fig. 40.1). This shortens operating time by limiting iliac artery and vein dissection to one side and preserves the contralateral iliac vessels should future re-transplantation be required.
The Pediatric Donor
Kidneys from pediatric donors (Fig. 40.1) are still regarded controversially with respect to long-term graft survival and function, particularly if originating from donors younger than 12 months of age. There is also a reluctance to separate pediatric kidneys for KT into two recipients. Recent studies have demonstrated excellent results with pediatric en bloc KT. A recent UNOS analysis demonstrated that long-term outcomes of pediatric en bloc KT from donors less than 5 years of age were superior to matched recipients of solitary, same age, pediatric KT, and SCD adult kidney recipients. The ongoing shortage of organs has prompted to explore the limits of acceptability of single KT from small pediatric donors into two recipients. Laurence et al. constructed a decision analysis model to predict the outcome in life years for patients with ESRD on the waiting list, depending on whether they received en bloc or solitary KT. Interestingly, at all recipient ages, the projected life years of both recipients of a solitary KT exceeded the projected life years of a recipient of an en bloc KT. Only recipients of solitary KT from donors weighing less than 10 kg had an estimated net loss of life years. Another UNOS review by Sureshkumar showed that the graft failure risk of solitary pediatric kidneys was consistently lower when the donor weight exceeded 10 kg. However, in this study, pediatric en bloc kidneys outperformed solitary pediatric kidneys for all donor weight groups. The Keyler review of UNOS data from 1995 to 2007 showed that the graft failure risk of solitary pediatric KT from donors weighing more than 35 kg was similar to ideal SCD KT from donors aged 18–39 without other risk factors, whereas solitary kidneys from pediatric donors weighing 10–35 kg performed more like non-ideal SCD KT. Overall, these studies indicate that more liberal transplantation of kidneys from pediatric donors may expand the donor pool. Solitary, rather than en bloc KT from donors weighing more than 10 kg offers more cumulative graft years and maximizes organ utilization without compromising outcomes and en bloc KT from donors weighing <5 kg has been favorable.
The Acute Renal Failure Donor
The rationale for using acute renal failure (ARF) kidneys is based on the observation that mechanisms of repair and regeneration do take place in the recipient. In 2006, Kumar et al. reported a series of 55 KT from ARF donors. Outcomes were compared with 55 concurrent and matched recipients of SCD kidneys and 55 concurrent and matched recipients of ECD kidneys. ARF kidneys were accepted from donors aged <50 years with no history of kidney disease and no chronic changes on pre-transplant biopsy. Three-year patient and graft survival was 90 and 90 % in the ARF group, 100 and 89 % in the SCD group, and 83 and 66 % in the ECD group. Biopsy-proven acute rejection rates were comparable among groups, although chronic allograft nephropathy (CAN) was significantly higher in the ECD group. Mean serum creatinine levels were 1.9, 1.9, and 2.2 mg/dl in the ARF, SCD, and ECD groups (SCD and ARF vs. ECD, p = 0.04). Kidneys from selected donors with ARF had comparable graft survival and function to non-ARF kidneys, despite a higher DGF rate; so transplantation of kidneys from ARF donors may help to safely expand the donor pool. In 2009, Kayler reviewed the 1995–2007 UNOS data to study the outcome and utilization of kidneys from deceased donors with ARF. For ECD recipients, the relative risk for graft failure significantly increased with rising serum creatinine level. Among potential SCDs, elevated serum creatinine level was a strong independent risk factor for kidney discard. However, when KT was performed, elevated donor terminal serum creatinine level was not a risk factor for graft loss. This study clearly underscores the fact that potentially transplantable kidneys are being discarded on the basis of elevated serum creatinine levels and that a more aggressive approach to transplanting kidneys from ARF donors may safely increase the number of deceased donor kidneys.
Living Donor and Paired Living Donor Kidney Transplantation
Many significant innovations and advances over the past decade have increased access to living donor KT. Technical progress in laparoscopic and, more recently, robotic donor surgery played a critical role for obvious reasons. From an immunological perspective, barriers represented by highly sensitized recipients and even ABO-incompatible donor-recipient pairs can be currently overcome by sophisticated desensitization protocols, which allow effective modulation of the immune response. Desensitization regimens, including high-dose intravenous immune globulin (IVIG), plasmapheresis, and rituximab, in highly sensitized recipients provide a significant survival advantage, more than doubling survival at 8 years. Although these protocols permit satisfactory early- to intermediate-term graft survival, both acute and chronic antibody-mediated rejection (AMR) remain significant issues, occurring in 20–50 % of HLA-incompatible KT and having a significant impact on graft survival and longevity. Emerging therapies to prevent and treat AMR include proteosome inhibitors aimed at plasma cells and modifiers of complement-mediated injury.
ABO-incompatible KT can nowadays also be performed successfully without high-intensity immunomodulation [9]. Current protocols involve a brief escalation in immunosuppression using plasmapheresis and IVIG with or without long-term B-cell suppression from splenectomy or anti-CD20 treatment with rituximab.
A newer development has been the growth of paired kidney donation as an alternative for KT candidates with willing and medically suitable live donors who cannot donate to their intended recipient secondary to an incompatibility of blood type or cross-match or both. In the most basic form of paired donation, the incompatibility problems with two donor-recipient pairs can be solved by exchanging donors. Using advanced software, several organizations have successfully completed paired kidney donations involving three or more pairs. Another permutation of paired kidney donor protocols is the “non-directed” or non-simultaneous, extended, altruistic donor chain, which is initiated by an altruistic donor and completed when the last paired donor in the chain donates to an unpaired recipient on the deceased donor waiting list. The proliferation of living donor exchange programs has increased transportation of live donor kidneys and has reduced geographic barriers to live donor KT. In spite of these exciting developments, however, the number of live donor KTs performed in the USA has actually declined in the past decade, in contrast to the experience in Eurotransplant. The more widespread application of living donation also has promoted the concept of preemptive kidney transplantation. Such approach has not only a great social advantage but even more an immunologic value allowing to markedly extent graft survival [10].
Robot-Assisted Transplantation
The Universities of Pisa and Chicago have recently shown that KT and also pancreas transplantation (PT) are feasible either by laparoscopy or laparoscopic aided robotic assistance. The rationale behind these initial experiences lay in that, when compared with conventional laparoscopy, the robot offers three-dimensional high-definition view, uses wristed instruments with seven degrees of freedom, and eliminates hand tremor. The surgical manipulative ability is further enhanced by the binocular stereoscopic steady viewing and by toggling among three operative arms to eventually improve the overall surgeon’s ability to operate within deep and narrow spaces. Although some of these advantages remain speculative, initial results already provide the rationale for further studies. Should this approach prove to be safe and effective in larger series, it may become an alternative to conventional open (maximally invasive) transplantation technique.
Organ Recovery and Regenerative Medicine
The marked extension of donor criteria recently renewed the interest for organ recovery/resuscitation laboratories aiming at improving the function of the “at-risk” recovered kidney grafts. Two major tendencies are under investigation, the hypothermic or normothermic perfusion. The judge is still out which method is best.
Indirectly the shortage of organs and the extension of donor criteria lead to a renewed interest for regenerative medicine. Many advances have already been made in the “kidney field.” The “creation and modulation” of allografts slowly but surely becomes a realistic approach allowing not only to expand in the future the donor pool but also to reach the final goal of transplantation, the “holy grail” of clinical operational tolerance [2].
Pancreas Transplantation
The Beginnings: From the 1960s to the End of Twentieth Century
Vascularized pancreas transplantation (PT) is currently the only definitive long-term treatment for selected patients with complicated type 1 or 2 diabetes (or those rendered diabetic by total pancreatectomy) that reliably restores normal glucose homeostasis without the need either for exogenous insulin therapy or close glucose monitoring. In addition, by achieving euglycemia, PT may not only eliminate the risk of either severe hypo/hyperglycemia but also prevent, stabilize, or even reverse progressive diabetic complications. PT can be performed either simultaneously with a kidney transplant (SPKT), following a successful KT (pancreas after kidney, PAKT) or prior to a KT as a pancreas transplant alone (PTA). The latter two categories are usually combined as solitary pancreas transplantation (SPT) because of similar outcomes. In the USA, 75 % of PT are performed as SPKT, 16 % as PAKT, and 9 % as PTA [11, 12]. The total number of PT steadily increased in the USA until 2004 but has since declined, particularly in the PAKT category. In contrast, the number of PTs performed outside of the USA has steadily increased in recent years and now accounts for the majority of PTs performed worldwide.
During the last decade, era analyses in the USA have demonstrated that deceased donor recovery rates and additions to the waiting list decreased; organ discard rates and waiting times increased; and the proportion of recipients who are older, black, have a higher BMI or are characterized as having type 2 diabetes all increased. Importantly, success rates for PT have steadily improved over time as a consequence of significant reductions in technical and immunologic failures. Appropriate donor and recipient selection, minimization of cold ischemia, and use of depleting antibody induction coupled with robust maintenance, usually tacrolimus-mycophenolate-based immunosuppression, are all of paramount importance to achieve success in PT. US recipients of primary deceased donor PT have a 1-year patient survival rate of more than 95 % in all three categories; unadjusted 5-year patient survival rates are 87 % in SPKT, 83 % in PAKT, and 89 % in PTA recipients; more than 70 % of patients are alive at 10 years post-transplant. One-year pancreas (insulin-free) graft survival rates are 85.5 % in SPKT (93 % kidney graft survival), 80 % in PAKT, and 78 % in PTA recipients, which translates to pancreas graft half-lives approaching 14 years in SPKT and 10 years in SPT recipients [11, 12].
The Standard Surgical Technique
The first PT, performed on December 16, 1966, at the University of Minnesota by Lillehei, was a segmental pancreas graft performed with duct ligation as an SPKT. The next 12 PTs performed at the same institute were whole-organ pancreatico-duodenal grafts with exocrine drainage diverted to a Roux-en-Y jejunal limb. After this initial experience, segmental pancreatic grafts were predominant in the late 1970s and early 1980s until newer techniques were introduced to safely manage the exocrine secretions. Since then, the vast majority of PTs have been performed as whole-organ grafts with a variable length of donor duodenum, while segmental pancreas grafts are now rarely used from deceased donors. However, this technique remains the only possibility in PT from living donors [13, 14]. According to Registry data, the majority of whole-organ PT has been performed with systemic venous delivery of insulin and either bladder (systemic-bladder) or enteric (systemic-enteric) drainage of the exocrine secretions.
Prior to 1995, more than 90 % of PTs were performed by the standard technique of systemic-bladder drainage, usually using a short donor duodenal segment conduit for exocrine drainage. Since 1995, the number of PTs performed with primary enteric exocrine drainage has increased dramatically and currently accounts for 91 % of SPKT, 89 % of PAKT, and 85 % of PTA cases in the USA. Over 80 % of enteric drained PTs are performed with systemic (iliac or vena cava) venous delivery of insulin, resulting in peripheral hyperinsulinemia. To improve the physiology, an innovative technique of intraperitoneal portal venous drainage using the superior mesenteric vein (SMV) was developed by Gaber et al. [15] and subsequently refined to a “retroperitoneal” approach by Boggi et al. combining portal venous delivery of insulin with enteric drainage of the exocrine secretions (portal-enteric technique (PET)) [16]. At present, however, portal-enteric drainage accounts for only 18 % of SPKT and PAKT and 10 % of PTA with enteric drainage [11]. Recent studies have reported excellent outcomes using either the recipient duodenum or stomach as the site for exocrine allograft drainage.
Although all techniques are similar in terms of long-term outcome, current philosophy dictates that the most appropriate technique to be performed is defined by both donor and recipient anatomy as well as the surgical team’s comfort level and experience. All PT techniques share common ground with respect to donor and recipient selection, organ recovery and preservation, bench preparation, and post-transplant management including immunosuppression. Most PTs are performed through a vertical midline abdominal incision, which preserves all possible options for transplantation of the pancreas, as well as eventual simultaneous placement of the kidney. Currently, the most common technique for arterial reconstruction of the dual-artery blood supply of the pancreas uses the bifurcating iliac artery from the donor as an interposition arterial “Y” graft. This graft can easily be anastomosed to the superior mesenteric (SMA) and splenic arteries. In the case of SPT, preemptive SPKT, history of thrombophilia or clotting disorder in the recipient, small or diseased donor or recipient vessels, prolonged cold ischemia, extended donor criteria, history of prior pancreas graft thrombosis, or abnormal intraoperative monitoring of coagulation status, perioperative systemic anticoagulation is warranted.
Advances in immunosuppression have played a pivotal role in the steadily improving results in PT. At present, 85–90 % of PT recipients receive antibody induction (70 % receive depleting antibody); nearly 80 % receive maintenance therapy with the tacrolimus/mycophenolate combination, and 40–50 % undergo steroid withdrawal without adverse consequences. Limited data with tacrolimus/sirolimus reveal excellent outcomes, whereas initial attempts with calcineurin inhibitor avoidance or minimization are less promising. One-year acute rejection rates have steadily decreased to the 5–20 % range depending on transplant category, case mix, and immunosuppressive regimen. Current 1-year rates of immunological pancreas graft loss are 6 % in PTA, 3.7 % in PAKT, and 1.8 % in SPKT recipients.
The Surgical Technique Refined: The Search for the Optimal Technique
During recent years there has been a continuous search towards the optimal endocrine and exocrine drainage technique.
Systemic Venous-Bladder Exocrine Drainage [or Systemic-Bladder Technique]
The introduction of the systemic-bladder technique (SBT) into clinical practice is regarded as a milestone in the history of PT because it dramatically lowered the exocrine complication rate and permitted a transition from the experimental to the clinical phase of PT. The graft is placed “head-down” in the pelvis. The vascular anastomoses are usually performed in an end-to-side fashion to the right external iliac vessels. The venous allograft outflow drains into the systemic venous system, thereby bypassing the portal system, thus causing systemic hyper-insulinemia and portal hypo-insulinemia. The donor duodenum (or pancreatic duct) is next anastomosed to the vesical dome (Fig. 40.2).
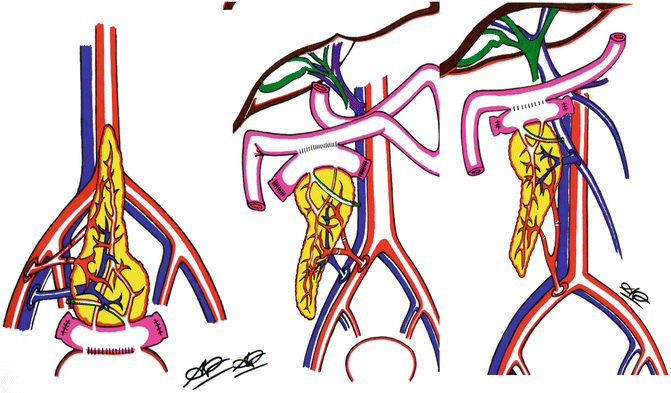
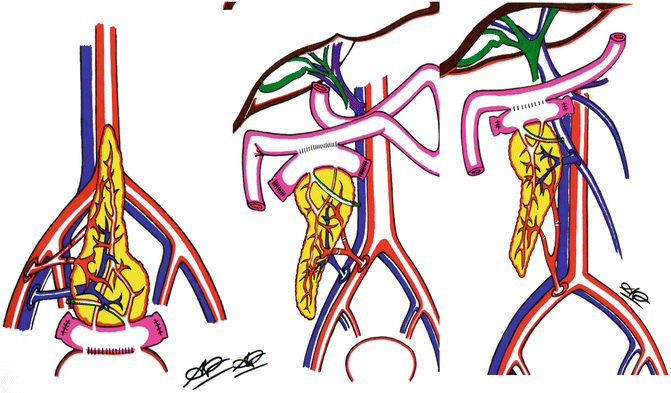
Fig. 40.2
Pancreas transplantation with bladder (left) or enteric drainage (mid and right). In the latter case, the portal vein can be implanted either on the iliac axis (mid cartoon) or on the SMV (right) (used with permission of Andrea Peloso, MD)
Safety and monitoring are the main advantages of the bladder diversion technique because exocrine leaks or pancreatitis can usually be diagnosed with standard imaging (including cystography) and managed with prolonged urethral catheter drainage. In fact, bacteria cultured from the graft duodenum are rarely involved in infections. Duodenal graft complications can be often managed conservatively with a low risk of graft loss or patient death. In addition, rejection can be more easily and timely diagnosed with monitoring of the urinary amylase concentration analysis and cystoscopy. The latter examination allows direct visualization of the duodeno-cystostomy and provides access for needle biopsy of either the duodenum or the pancreas allograft. The drainage of pancreatic enzymes into the bladder as well as systemic venous delivery of insulin have also disadvantages because it creates a non-physiologic condition associated with unique urologic and metabolic complications. These include hyperchloremic metabolic acidosis secondary to bicarbonate loss, dehydration secondary to fluid losses through the urine, recurrent urinary tract infections, dysuria, hematuria, cystitis/urethritis, and recurrent episodes of graft pancreatitis secondary to reflux of urine into the ductal system of the graft. In about 20–25 % of cases, enteric conversion is performed to eliminate persistent or recurrent urologic, metabolic, or infectious complications. Currently, SBT is used selectively but has not been abandoned because it is a time-honored technique with excellent long-term outcomes. It is used more commonly in SPT in order to allow monitoring of urine amylase levels and when there are concerns about the preservation or perfusion of the duodenal segment because leaks are easier to manage and have lower morbidity and a better chance to heal with bladder drainage.
Systemic Venous-Enteric Exocrine Drainage [or Systemic-Enteric Technique]
Because of the success of enteric conversion coupled with advances in donor management, organ preservation, immunosuppression, diagnostic methodology, antibiotic strategies, and overall medical management, a transformation in PT occurred from SBT to systemic-enteric technique (SET) in the mid-1990s. Since then the majority of PTs are performed using the SET with a variable length of duodenum. The SET is the most versatile technique because it permits either cephalad or caudal orientation of the pancreas with exocrine drainage into virtually any part of the mid-gastrointestinal (GI) tract (stomach, duodenum, jejunum, or ileum). The vascular anastomoses are similar as in SBT (end-to-side anastomoses to the iliac vessels or inferior vena cava/aorta). A number of techniques of performing the enteric anastomosis have been described either with or without a diverting Roux-en-Y limb including side-to-side or end-to-side anastomoses. The enteric anastomosis can be stapled or hand-sewn. Some surgeons prefer the hand-sewn anastomosis secondary to a higher bleeding risk of the stapled enteric anastomosis, especially when localized more proximal in the GI tract.
Portal Venous-Enteric Exocrine Drainage [or Portal-Enteric Technique]
The portal vein of the pancreas graft is anastomosed end to side to a major tributary of the SMV using a standard vascular running technique with polypropylene sutures. The dissection of the SMV can be performed with either an anterior, intraperitoneal, or a lateral, retroperitoneal, approach. In the former, the donor iliac artery bifurcation graft is brought through a window created in the distal ileal mesentery and anastomosed end to side to the right common iliac artery. In the latter, no window is necessary for the artery whereas a window may be needed in the right colon mesentery for the enteric anastomosis. The pancreas will be oriented vertically with the head of the pancreas directed cephalad either in the mid-abdomen or right para-colic region depending on the approach to the SMV (Fig. 40.2). The enteric anastomosis is more commonly created directly into an adjacent bowel loop that is not excluded from the transit of intestinal contents or to a Roux-en-Y limb that is diverted from the enteric stream with or without a venting jejunostomy. Less frequently, the enteric anastomosis can also be created in an omega loop or directly into the native duodenum or stomach, which may allow easier access for endoscopic surveillance and biopsy. Complications of enteric drainage (SET or PET) include prolonged ileus, small bowel obstruction, enteric leak, infected peri-pancreatic fluid collections, GI bleeding, and intra-abdominal abscess formation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








