CHAPTER 61 ABDOMINAL COMPARTMENT SYNDROME, DAMAGE CONTROL, AND THE POST-TRAUMATIC OPEN ABDOMEN
Major contributors to improved survival in the field of trauma and surgical critical care over the past decade include the early recognition and preventive strategies in the management of the abdominal compartment syndrome and the systematic, staged surgical approach to the trauma patient in extremis called damage control. However, in our efforts to cure one disease, we have created another: the post-traumatic open abdomen, defined as a large postoperative ventral hernia with the abdominal viscera covered by a temporary dressing or closure.
ABDOMINAL COMPARTMENT SYNDROME
The effects of increased intra-abdominal pressure were first described in 1863 by Marey and Burt, who reported the relationship between intrathoracic pressure and elevated intra-abdominal pressure. However, it was not until the 1980s that Kron and Richards coined the term “abdominal compartment syndrome” (ACS).1,2 They reported a separate series of patients that developed a tense, distended abdomen with elevated pulmonary artery pressures and increased intraabdominal pressures postoperatively despite normal mean arterial blood pressure and cardiac performance. All of these patients improved with re-exploration and abdominal decompression.
Intra-abdominal hypertension and the abdominal compartment syndrome are not synonymous. ACS is a late manifestation of uncontrolled intra-abdominal hypertension produced by ongoing ischemia and splanchnic hypoperfusion and the resuscitative measures to counteract the hemorrhagic shock state (Figure 1).
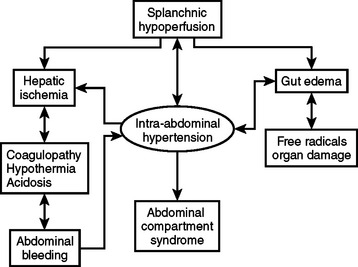
Figure 1 A cycle of ischemia producing intra-abdominal hypertension and the abdominal compartment syndrome.
(Reproduced with permission from Michael Rotondo, MD.)
Table 1 lists the risk factors associated with ACS. Mortality from the fulminant abdominal compartment syndrome has been reported to be as high as 67%.
Table 1 Etiology of Abdominal Compartment Syndrome: Who Is at Risk?
In addition to the accumulation of blood within the perineal cavity, other factors may contribute to occupying space within the abdominal cavity. This can occur by any shock-induced visceral ischemia and reperfusion edema including major injuries outside the abdominal cavity. Several recent reports have described this “secondary abdominal compartment syndrome,” which occurs most frequently with major pelvic and long bone fractures, and hemorrhagic chest injuries. However, secondary ACS can occur in any setting associated with hemorrhagic shock.3–5
Balough and associates reviewed patients with major torso trauma and found that both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiorgan failure. Fourteen percent of these patients develop abdominal compartment syndrome, all of which require aggressive resuscitation using crystalloid, blood, and blood products early in their initial management in the emergency department. Therefore, the current emphasis in critical care management of the severely injured patients focuses on identification of predictive factors for the development of ACS and the recognition of intra-abdominal hypertension and treatment before full development of the syndrome.7–12
Intra-abdominal hypertension affects multiple organ systems in a graded fashion. The deleterious consequences appear gradually, and the adverse effects of elevated intra-abdominal pressure occur at lower levels than previously thought and manifest before the development of the fulminant syndrome (Table 2).
Table 2 Effects of Intra-Abdominal Hypertension on Organ Systems
| Head | ↑ Intracranial pressure |
| ↓ Cerebral perfusion pressure | |
| Heart | ↓ CO |
| ↓ Venous return | |
| ↑ Pulmonary artery occlusion pressure and central venous pressure | |
| ↑ Systemic vascular resistance | |
| Lungs | ↑ Peak inspiratory pressure |
| ↑ Pulmonary artery wedge pressure | |
| ↓ Dynamic compliance (Cdyn) | |
| ↑ Arterial oxygen pressure (PaO2) | |
| ↑ Arterial carbon dioxide pressure (PaCO2) | |
| ↑ Intrapulmonary shunt (Qsp/Qt) | |
| ↑ Fraction of dead space to total expired tidal volume (VD/VT) | |
| Liver | ↓ Portal flow |
| ↓ Mitochondria | |
| ↑ Lactate | |
| Kidney | ↓ Urine output |
| ↓ Renal flow | |
| ↓ Glomerular filtration rate (GFR) | |
| Intestines | ↓ Celiac flow |
| ↓ Superior mesenteric arterial (SMA) flow | |
| ↓ Mucosal flow | |
| Abdomen wall | ↓ Compliance |
| ↓ Rectus flow |
The classic picture of ACS includes a patient with a tense, distended abdomen and ventilatory insufficiency including hypoxia and hypercarbia, as well as increased peak inspiratory pressures. Progressive oliguria occurs despite adequate mean arterial pressure and cardiac output. This is followed by decreased cardiac performance and subsequent cardiovascular collapse unless treatment is instituted immediately (Table 3).
Table 3 Abdominal Compartment Syndrome: Classic Clinical Picture
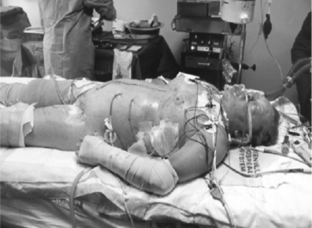
Figure 2 illustrates a high-risk patient for ACS: tense abdomen, respiratory failure, and progressive oliguria in a multitrauma patient requiring aggressive resuscitation for hemorrhagic shock.
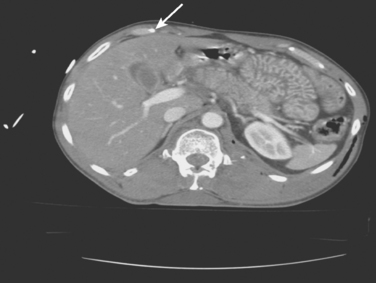
Common computed tomography findings in this patient population include extraperitoneal hematoma and/or extravasation, intra- and retro-peritoneal edema, and “shock bowel” defined as an intense mucosal enhancement producing a prominent, feather-like appearance to the small intestines (Figure 3).
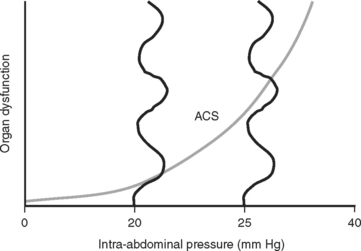
The consensus at the World Congress on Abdominal Compartment Syndrome defines this disease entity as persistent bladder pressures over 20 mm of mercury with the new onset of organ failure. Figure 4 demonstrates the progression of organ dysfunction as intra-abdominal pressure increases over this level. Once defined, ACS mandates immediate decompressive celiotomy (Figure 5).
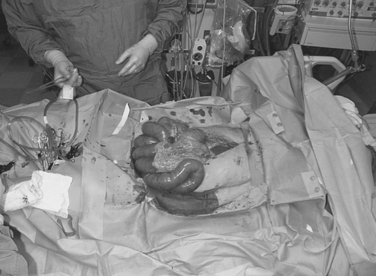
Figure 5 Bedside decompressive celiotomy for abdominal compartment syndrome (note massive small bowel edema).
This often rapidly reverses all the adverse affects of increased intra-abdominal pressure and dramatically improves oxygenation and pulmonary compliance, returning peak inspiratory pressures toward normal and promptly reversing the oliguria with a brisk diuresis of resuscitation fluids. Before decompression, all attempts to correct acid–base and electrolyte disturbances including potassium, magnesium, and calcium may avoid cardiac dysrhythmias after decompression. A respiratory therapist should also be immediately available to readjust ventilatory settings to prevent additional pulmonary barotrauma.
DAMAGE CONTROL
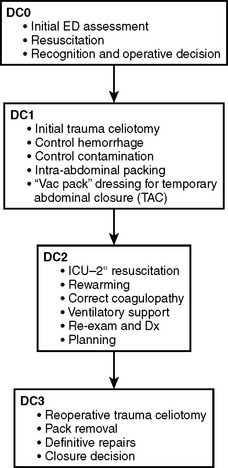
An abbreviated celiotomy and intra-abdominal packing for hemorrhage control in the abdomen was first described by Stone13 in 1983. However, the term “damage control” was first coined by Rotondo and Schwab at the University of Pennsylvania in 1993. The current management scheme has been revised to include four stages (Figure 6).14–16
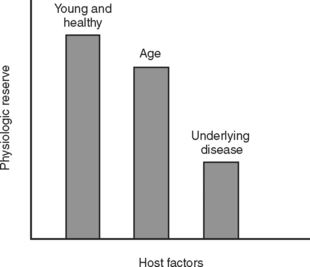
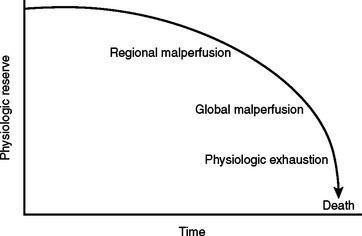
During the initial trauma celiotomy (DC1), it is important to determine each patient’s physiologic reserve in order to make appropriate operative decisions. Physiologic reserve is defined as an individual’s unique ability to tolerate injury. It is a function of several host factors including age, gender, pre-existing disease, genetics, and immunocompetence (Figure 7). As physiologic reserve becomes depleted during hemorrhagic shock, regional and then global malperfusion occur, leading to physiologic exhaustion and subsequent death (Figure 8).11
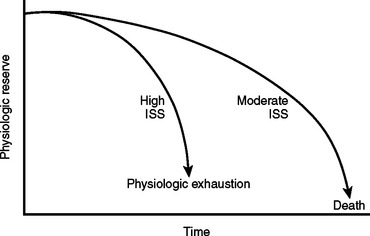
Mortality associated with damage control procedures ranges between 25%–60%. Each patient responds and reacts differently to stress and injury, and each individual has a limited amount of compensatory reserve until physiologic exhaustion is reached. Additionally, the extent of injury severity determines the slope leading to physiologic exhaustion (Figure 9).
During the initial trauma celiotomy, the surgeon must recognize the need for immediate control of major hemorrhage and contamination with maximum replacement of coagulation factors including platelets, fresh frozen plasma, and cryoprecipitate, and in certain circumstances, factor VIIa for microvascular bleeding. Intraoperative monitoring of temperature, arterial blood gases, and volume of resuscitative fluids are important in determining whether a patient is descending down the physiologic curve toward physiologic exhaustion. In addition, for patients suffering hollow viscus injuries, contamination is controlled with linear staples to transect the bowel ends and leave them in discontinuity until a later stage of damage control management.
Table 4 lists clinical parameters that should prompt the surgical team to initiate damage control maneuvers, abort the operation, and return to the ICU for resuscitation and restoration of reserve. Asensio17,18 recommends instituting damage control early, well before reaching the upper limits of physiologic exhaustion, and describes statistically validated criteria. Using these statistically validated intraoperative predictors of instituting damage control, patients with post-traumatic open abdomen incurred less hypothermia and fewer postoperative complications including intra-abdominal abscess and fistula formation. Patients with early damage control were also subjectively noted to have less bowel edema and were able to undergo definitive abdominal wall closure during their initial hospital stay.
Table 4 Clinical Guidelines to Abort Initial Trauma Celiotomy and Initiate Damage Control Maneuvers
| Hypothermia | <35° C |
| Acidosis | pH <7.2 |
| Base deficit (BD) ≥–8 | |
| Lactate ≥4 | |
| Coagulopathy | Activated partial thromboplastic time (aPTT) >60 |
| International normalized ratio (NR) >1.6 | |
| Ongoing resuscitation | Persistent shock systolic blood pressure <90 |
| >10 liters crystalloid | |
| >10 units packed red blood cells | |
| Operative time | >60–90 minutes with abdominal cavity opened |
Temporary Abdominal Closure
A TAC is defined as any technique that contains the abdominal viscera during the acute phase of care. Indications for creating a TAC are listed in Table 5.
Table 5 Indications for Temporary Abdominal Closure
< div class='tao-gold-member'> Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|