Chapter 12 A ‘functional pathology of the motor system’1 involves a pattern generating mechanism underlying most spinal pain disorders
Janda2 said: ‘the biological function of pain is that it signals bad or harmful function; it is the motor system’s way of protecting itself when overstressed. Pain will sooner or later force us to change our motor behavior. Pain may be considered as the major and most frequent sign of impaired function of the motor system. It is not a ‘disease’ as Western medicine chooses to see it. The patient’s pain can help us unravel his functional problems and can also act as the incentive for him to change the bad movement habits that have generally created it.
Lewit’1,3 adopted the term ‘functional pathology of the motor system’ to encompass the most important functional changes together with the reflex changes they produce. The impaired function may be reflected anywhere in the motor system; however, roughly speaking, there are three basic yet functionally interdependent levels where it is seen:2 the central nervous system (CNS) corticosubcortical motor regulatory centers; the muscles; and finally the joints. Altered afference produces altered motor output and the muscular level represents perhaps the most exposed part of the system’2 (see Ch. 7). Neuromuscular control of the spine is complex and involves the interaction of all levels of the motor system.
Whilst a clinically useful and compelling paradigm, it is only more recently that there has been more interest and emerging evidence which in principle supports aspects of the functional approach proposed by Janda and Lewit.4–8
Altered loading stresses through the functional spinal unit (FSU) affects local, regional and general neuromuscular responses
The segmented spinal column houses the main nerve trunks between the brain and the periphery. Its functional wellbeing ensures the health of the entire nervous system. The nervous system is a continuous tissue tract which continually glides, slides and stretches as it adapts to the movements it orchestrates.9,10 When the activity level between the two muscle systems is out of balance, postural and kinematic patterns of movement alter and the whole spine suffers as the compression and tension loading stresses across it change. Altered neuromuscular control is reflected in essentially four ways:
Altered postural responses within the column
Altered alignment and loading patterns in one part of the spine will affect those in adjacent and more removed segments. While resulting from changed neuromuscular control they also result in the need for further postural compensations being brought to bear in the system. An example is poor spatial control of the pelvis alters the alignment and control of the lumbar lordosis and necessitates muscular ‘holding’ patterns higher up the torso in order to support the column and head upright. The ability for the pelvis to contribute to postural control is reduced.11 Some segments and regions are loaded in more tension, others in compression. Reduced ability in finely adjusting and controlling intersegmental movement means individual segments become further compromised. Every spinal segment is susceptible and symptoms arise depending upon the individual circumstances. However, the discussion here will focus on the lumbar spine as most of the literature pertains to this region.
Habitual provocative posturomovement strategies
Neurologically we get used to firing some muscles repeatedly and forget to use others as we repeat various less ideal movement patterns again and again. A good example is when bending forward the action principally occurs by locking the knees and relying on the hamstrings and obturator group with poor contribution from the antagonists in the controlling pelvic force couple – the transversus, iliacus and psoas and other LPU muscles. The hamstring hyperactivity limits posterior pelvic shift and further defacilitates the antagonistic contribution (Fig. 12.1). The habit of crossing the arms in front of the chest fires up the pectorals and serratus contributing to ‘dome’ development and disturbing cervical and shoulder girdle myomechanics.
Altered segmental muscle function
The passive viscoelastic structures within each FSU (Ch. 6, Part A) enjoy a rich sensory and autonomic innervation enabling them to transmit proprioceptive and nociceptive information.12 The reflexive feedback control of local muscular contraction consists of afferents in the ligaments, disc and facet joint capsules, spinal interneurons and selected trunk muscles.13 Altered alignment and movement stresses through the segment can induce progressive creep and hysteresis in the ligaments, the development of joint laxity reduced joint stability and the risk of injury.14 Feline studies have shown that the induced creep in the viscoelastic tissues also desensitizes the mechanoreceptors and results in a dramatic loss of reflexive muscular activity and stabilization.15 The induced laxity only showed partial recovery with rest periods twice as long as the loading duration and recovery of reflexive muscular activity follows the recovery of laxity in the viscoelastic structures.16 More prolonged static loading in lumbar flexion (20 minutes) produced the initial sharp decrease in multifidus activity followed by spasms.17 Full recovery of reflexive multifidus activity and viscoelastic tension did not occur for up to 24 hours. Static constant loading in flexion not only results in a complex neuromuscular disorder18 but also importantly the time dependent development of local inflammation.19,20 Repetitive static loading into flexion increases the likelihood of a cumulative neuromuscular disorder.21 Injecting porcine facet joints with saline reduced paraspinal muscle activity.22 Beith23 showed delay in the short latency stretch reflex in multifidus but not in rectus abdominus or internal oblique in subjects with CLBP. In a porcine study, Hodges et al.24 found rapid atrophy in multifidus 3 days after experimentally inducing an acute disc injury at L3/4 or an L3 nerve root injury. The changes after the disc lesion produced single segment atrophy and they concluded this may be due to disuse following reflex inhibitory mechanisms. Nerve root injury reduced the cross sectional area over three segments.
Altered multisegmental muscle function
Local segmental irritation can either decrease or more usually increase activity in SGMS muscles which receive innervation from that segment(s). These large muscles span numerous segments and being large torque producers with domineering behavior, can act as ‘yankers’ further disturbing axial control (see Ch. 5) This may involve muscles within the torso such as the erector spinae or more peripheral muscles such as the hamstrings which then further influence pelvic control. Eccentric contractions and lengthening behavior in patterns of movement appear to be more difficult in these muscles.
Clinically increased tension in the hamstrings in association with back pain is well appreciated; however, the fact that the same mechanism can effect changes in other peripheral muscles is nor so well known. Upledger25 suggests mobilizing the upper lumbar spine levels can relax spasm in iliacus. A statistically significant relationship between evident trigger points in the upper trapezius and cervical dysfunction at C3&4 has been reported.26 Dishman and Bulbulian27 demonstrated spinal mobilization and manipulation produced a profound yet transient attenuation of reflex excitability in the gastrocnemius. Sacroiliac and spinal manipulative therapy (SMT) has been shown to generate reflex activation of upper and lower limb muscles28 and to decrease quadriceps inhibition in patients with anterior knee pain.29 SMT to L4/5 has also been shown to change superficial abdominal muscle recruitment in postural activity in people with low back pain but not in controls.30
The potent influence of spinal segmental irritation as the driver of much limb muscle ‘tightness’ seems little appreciated in the clinical community. It is suggested that ‘central axial drive’ of peripheral muscle tightness or hyperactivity largely contributes to the pattern generating mechanism responsible for symptom development seen in many ‘fitness industry’ participants and particularly so in most functional spinal pain disorders (see ‘The hamstrings/hip conundrum’ p. 286).
Whether a muscle is under-firing or over-firing will thus variably depend on CNS influences, local segmental reflex influences as well as the habitual strategies chose in everyday posturomovement activity. Further, disrupted sensory feedback appears to have a greater effect upon eccentric control than concentric control.31
Altered loading stresses of any joint in the body will generally result in reactive inflammatory changes. It is important to recognize that a stiff spinal joint readily becomes an inflamed joint as does a joint that is relatively over mobile. Arguably, in the clinical realm, stiff joints appear to cause more abrasive neurally related symptoms than over-mobile joints. A joint can be stiff in all or some of its available ranges. A joint which is over stressed into flexion with probable creep/hysteresis will generally be stiff into extension and related movements particularly those through the junctional regions. The middle lumbar and cervical segments risk becoming overstressed into both flexion and extension, potentially developing a structural or ‘functional instability’. Any inflammatory change within the FSU is liable to create neural irritation to some degree which in the early stages will be sub-clinical, manifesting as altered facilitation or inhibition of muscles which derive their innervation wholly or partly from that segment. The influence of segmental movements on muscle activity can be appreciated in a normal study which showed that moderate central pressures applied to L3 when the subject was prone produced statistically significant reductions in erector spinae EMG.32 Janda33 notes that when the intraarticular pressures change, the irritability of the muscles in the vicinity changes. Traction or separation of the joint surfaces facilitates the flexor groups, whereas compression of the articular surfaces in the joint’s longitudinal axis facilitates the extensors.
The altered local and multisegmental muscle function results in altered afference to the CNS which in turn results in changed motor output from the CNS. ‘This two way traffic of cause/effect/cause’34 further adds to the pattern generating process in the developing neuromusculoskeletal dysfunctional disorder.
Altered loading stress through the FSU creates the conditions for neural irritation creating local and referred pain and other epiphenomena
The radiculopathic model for the genesis of many chronic pain syndromes is well understood by experienced clinicians. Irritation or damage to a peripheral nerve invariably at the spinal nerve root leads to muscle shortening, autonomic changes and sometimes pain in the dermatomal, myotomal and sclerotomal target tissues supplied by that segmental nerve.35 Simple inflammation rather than structural changes are more often the cause –‘biologically or ergonomically triggered neurogenic inflammation.’36
Each FSU intimately encases and contributes to the protection of the spinal cord and the spinal nerve root as it exits through the intervertebral foramen (IVF). The nerves are numbered according to the vertebra beneath which they lie. Thus, the L1 spinal nerve lies below the L1 vertebra in the L1/2 IVF:12 Centrally each spinal nerve is connected to the spinal cord by a dorsal and ventral root (Fig. 12.2).
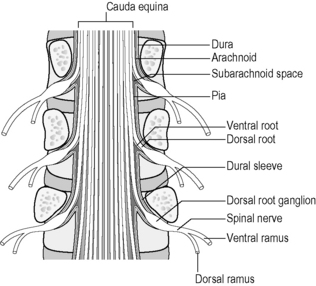
Fig 12.2 • Lumbar spinal nerve and its relations in the central and lateral canal after Bogduk 198712.
The dorsal root of each spinal nerve transmits sensory fibres from the spinal nerve to the cord. The ventral root largely transmits motor fibres from the cord to the spinal nerve but may transmit some sensory fibres.12 The ventral roots of L1 and L2 spinal nerves additionally transmit preganglionic, sympathetic, efferent fibres.12 The spinal cord terminates in the central vertebral canal opposite L1/2 but this can be as high as T12/L1 or as low as L2/3. The lower lumbar, sacral and coccygeal roots are all enclosed together within the dural sac and descend together as the cauda equina.12 (Fig. 12.3).
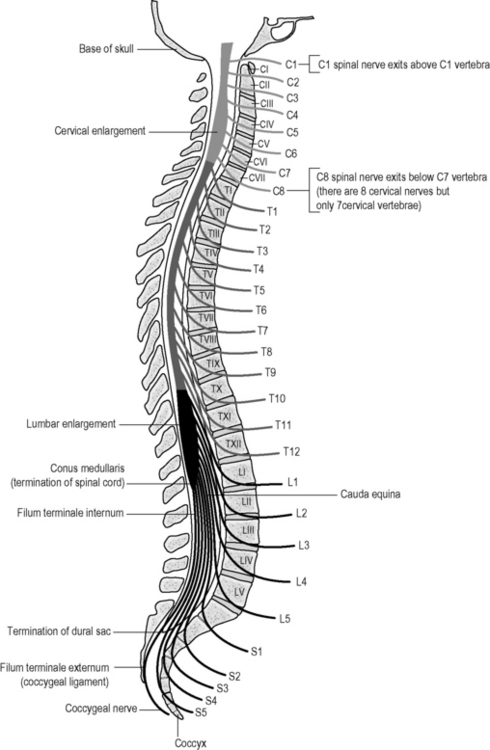
Fig 12.3 • The spinal nerves in relation to the vertebrae. Note the conus is adjacent to the thoracolumbar junction. Irritation of these levels potentially influences a number of nerves.
The dorsal root ganglion contains the cell bodies of the sensory fibres in the dorsal root and lies immediately proximal to its junction with the spinal nerve within the intervertebral foramen. The spinal nerve root sleeve is surrounded by circumferential layers of connective tissue which indirectly bind the nerve to the margins of the IVF but importantly, mainly to the capsule of the facet joint dorsally.12 This helps explain how clinically, a swollen or thickened facet joint can cause radicular symptoms.
Peripherally, just outside the IVF, each spinal nerve divides into a larger ventral ramus and a smaller dorsal ramus.12
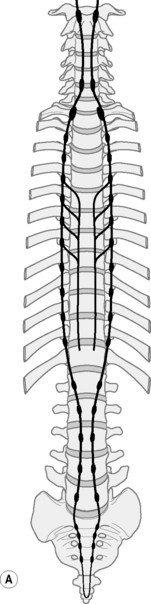
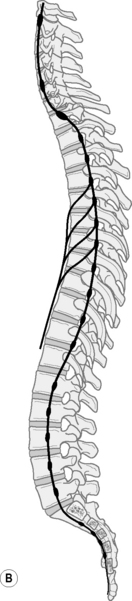
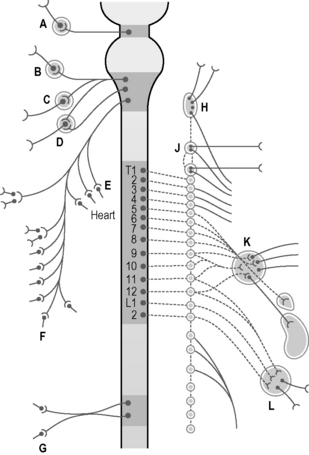
The autonomic nervous system must adapt to body movements if it is to function properly. Full utilization of bodily movement ensures its flexibility and health. The sympathetic trunk lies anterior to the whole column and in the thorax it is also attached to the head of the ribs9 (Fig. 12.4). In the lumbar spine the trunks lie next to the attachment of psoas.12 Note in Figure 12.5 that the ‘sympathetic outflow’ extends from T1–L2 via the thoraco/lumbar somatic nerves; while the parasympathetic system utilizes the cranial nerves III, VII, IX, X and sacral somatic nerves S234 for its pathways – known as the ‘craniosacral outflow’. Both the sympathetics and parasympathetics transmit pain; however, concerning pain in the lower body, the sympathetics will refer to dermatomes associated with the lower sympathetic trunk (T10–L2),34 while parasympathetics refer to dermatomes associated with S2, and S3 (and S4) segments.37 In the upper body, the afferent sympathetic pathways to the head and neck travel with the segmental nerves T1–5, and those to the upper limb, T2–10.34
An unhealthy posture of increased thoracic and lumbar kyphosis and cervical extension is likely to place altered tension on the sympathetic trunk.9 Mobilization to L4/5 has demonstrated significant changes in peripheral sympathetic activity in skin conductance.38 Impairment of the sympathetic system could be an etiologic cause or perpetuating consequence for the development of active trigger points.39
Local and referred pain
Grieve34 states ‘in all pain states, the somatic and autonomic nervous systems are activated in a variety of manifestations and degree. Considerations of spinal pain and referred pain in spinal conditions should include attention to visceral reflex phenomena also’. Similarly, Lewit1
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree