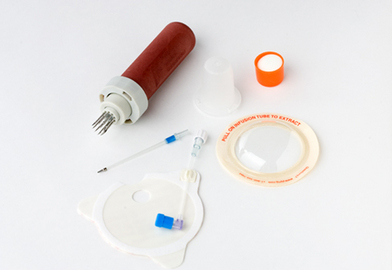
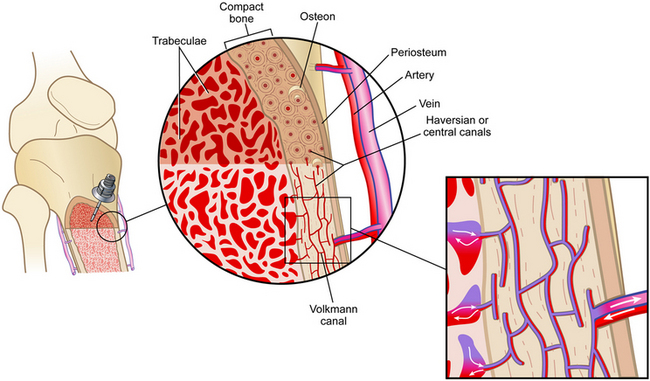
PROCEDURE 84 Intraosseous access is indicated when intravenous access cannot be obtained or cannot be obtained in a timely manner (within 90 seconds10) and access to venous circulation is needed for the administration of medications or fluids. • Intraosseous (IO) access is a safe and reliable access point into the noncollapsible marrow cavity that allows direct access to the venous circulation. • The Volkmann’s canals that are located throughout the bone connect with the medullary canal and the blood vessels of the periosteum (Fig. 84-1). When medications and fluids are introduced into the medullary canal, they flow through the vascular plexi directly into the vascular system.6 Figure 84-1 IO Circulation. (From Day MW: Intraossesous devices in the adult trauma patient,Crit Care Nurs. In press.) • Insertion devices are available for insertion of IO needles.6 These devices include the bone injection gun (BIG; Waismed, a Persys Medical Co, Houston, TX; Fig. 84-2), the FAST1 adult intraosseous infusion system (Pyng Medical Corp, Vancouver, BC, Canada, Fig. 84-3), and the EZ-IO (Vidacare Corp, Shavano Park, TX; Fig. 84-4). These three devices are approved by the US Food and Drug Administration (FDA) for IO access in adult patients. Two of the devices (BIG, EZ-IO) use a specially designed needle with a stylet or trocar. The third device (FAST1) uses a metal-tipped plastic catheter. • In adults, the available IO access sites, depending on the specific device and following each manufacturer’s guidelines, include: • IO blood can be used for many laboratory tests, including typing and screening, electrolyte values, chemistries, blood gas values, drug levels, and hemoglobin levels.2 However, specimen samples from the marrow have a lower correlation to serum levels after 30 minutes of resuscitation.3 In addition, drawing of blood from an IO device may not be recommended by specific manufacturers and has the potential of occluding the device. • The onset of action for medications is similar to that of intravenous (IV) medications.13 However, administration via the IO route may result in lower serum concentrations versus the IV route for the following medications: ceftriaxone, chloramphenicol, phenytoin, tobramycin, and vancomycin.5 • Marrow-toxic medications should not be infused via the IO route.8 • All resuscitation medications, isotonic fluids, and blood products may be given via the IO route2,3,5,14; however, myonecrosis has been reported with the infusion of hypertonic saline solution via the IO route.10,12
Intraosseous Devices
PREREQUISITE NURSING KNOWLEDGE
 Tibial plateau: 1 to 2 cm distal to the tibial tuberosity3,5,8
Tibial plateau: 1 to 2 cm distal to the tibial tuberosity3,5,8
 Distal tibia: 1 to 2 cm above the medial malleolus7
Distal tibia: 1 to 2 cm above the medial malleolus7
 Sternum: 1.5 cm below the sternal notch5,8
Sternum: 1.5 cm below the sternal notch5,8
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


84: Intraosseous Devices