PROCEDURE 56 Ventricular assist devices are used for cardiogenic shock and postcardiotomy support to allow for myocardial recovery, for bridge to cardiac transplantation, and for destination therapy (permanent implantation) in patients with New York Heart Association class IIIB or IV heart failure who are on optimal medical therapy and are not eligible for cardiac transplant.3–6,9,14 • Understanding of the normal anatomy and physiology of the cardiovascular, peripheral vascular, and pulmonary systems is important. • Understanding of the management of heart failure is essential. • Knowledge of the principles of hemodynamic monitoring, cardiopulmonary bypass, electrophysiology and dysrhythmias, and coagulation is needed. • Clinical and technical competence related to use of ventricular assist devices (VADs) is necessary. • Advanced cardiac life support knowledge and skills are needed. • Complications of VAD therapy include, but are not limited to, bleeding, cardiac tamponade, right ventricular failure with univentricular support, hepatic dysfunction, pulmonary dysfunction, renal dysfunction, infection, cerebral infarcts, thrombosis, embolism, and VAD malfunction.3,6 • Effective cardiac assistance with the VAD is affected greatly by preload, afterload, right ventricular failure, cardiac tamponade, and cardiac dysrhythmias; the interaction between the patient and the device requires close monitoring. • The device is implanted surgically in the operating room. Specific information concerning controls, alarms, troubleshooting, and safety features is available from each manufacturer and should be read thoroughly by the nurse before use of the equipment. Please refer to the operator’s manual for all systems for more detail. • Indications for VAD therapy include the following4,6, 9,10, 14: • Relative contraindications of VAD therapy include the following: • Psychosocial and cognitive conditions may limit the use of a VAD except in bridge to recovery because the patient needs to have the cognitive skills to manage the VAD. • Ventricular assist devices are pulsatile or nonpulsatile. • ABIOMED BVS 5000 circulatory support system (Fig. 56-1)2: Figure 56-1 ABIOMED BVS 5000 System. (From Dixon JF, Farris DD: The ABIOMED BVS 5000 system, AACN Clin Issues Crit Care Nurs 2:552-561, 1991.) • ABIOMED AB 5000 ventricle1 (Fig. 56-2): • HeartMate XVE (extended lead vented electric) left ventricular assist system (LVAS):17 • Thoratec Paracoporeal Ventricular Assist Device (PVAD):18
![]() Ventricular Assist Devices
Ventricular Assist Devices
PREREQUISITE NURSING KNOWLEDGE
 Inability to wean from cardiopulmonary bypass
Inability to wean from cardiopulmonary bypass
 New York Heart Association class IIIB or IV status in a patient whose condition does not respond to optimal medical therapy and who is not a transplant candidate
New York Heart Association class IIIB or IV status in a patient whose condition does not respond to optimal medical therapy and who is not a transplant candidate
 Body surface area (BSA) less than 1.3 m2 (ABIOMED BVS 5000 or AB 5000 ventricle; Abiomed Inc, Danvers, MA)1–3
Body surface area (BSA) less than 1.3 m2 (ABIOMED BVS 5000 or AB 5000 ventricle; Abiomed Inc, Danvers, MA)1–3
 BSA less than 1.5 m2 (HeartMate XVE left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
BSA less than 1.5 m2 (HeartMate XVE left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
 BSA less than 1.2 m2 (HeartMate II left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
BSA less than 1.2 m2 (HeartMate II left ventricular assist device [LVAD; Thoratec Corporation, Pleasanton, CA]11,12)
 Renal or liver failure unrelated to cardiac incident
Renal or liver failure unrelated to cardiac incident
 Comorbidity that limits life expectancy to less than 3 years
Comorbidity that limits life expectancy to less than 3 years
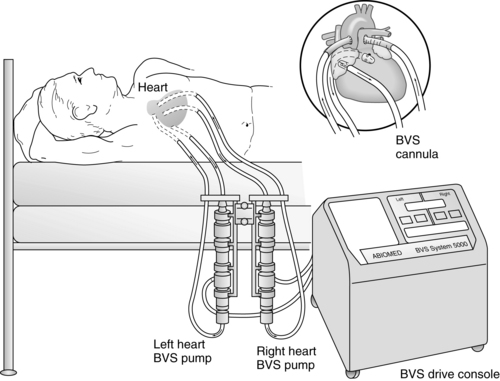
 The ABIOMED BVS 5000 is an extracorporeal, pneumatically driven pump capable of delivering short-term (less than 3 weeks) left, right, or biventricular support.
The ABIOMED BVS 5000 is an extracorporeal, pneumatically driven pump capable of delivering short-term (less than 3 weeks) left, right, or biventricular support.
 The drive console controls systole by delivering air into the lower rigid plastic pumping chamber, displacing blood from the blood sac. Blood drains passively from the patient’s atrium into the atrial chamber of the blood pump. When the atrial chamber of the blood pump is full and the pressure inside the atrial chamber exceeds the pressure inside the ventricular chamber, the trileaflet valve opens, allowing blood to flow into the ventricular chamber of the blood pump. Blood pump diastole is completed as soon as the ventricular chamber is filled with 100 mL. The diastolic filling time is adjusted automatically to changes in the patient’s preload to ensure the ventricular chamber is filled to capacity (100 mL).
The drive console controls systole by delivering air into the lower rigid plastic pumping chamber, displacing blood from the blood sac. Blood drains passively from the patient’s atrium into the atrial chamber of the blood pump. When the atrial chamber of the blood pump is full and the pressure inside the atrial chamber exceeds the pressure inside the ventricular chamber, the trileaflet valve opens, allowing blood to flow into the ventricular chamber of the blood pump. Blood pump diastole is completed as soon as the ventricular chamber is filled with 100 mL. The diastolic filling time is adjusted automatically to changes in the patient’s preload to ensure the ventricular chamber is filled to capacity (100 mL).
 The vertically aligned pneumatic blood pumps are adjusted to optimize flow. The blood flow from the patient to the blood pump depends on the console used. The BVS 5000t (transport) console and the AB 5000 console allow for vacuum-assisted filling of the chamber. Filling of the pumps depends solely on gravity when the BVS 5000 or BVS 5000i (high-flow) consoles are used. The top of the blood pump should be between 0 and 10 inches below the level of the patient’s atria when a 42 Fr atrial cannula is used and 4 to 14 inches when a 32 Fr or 36 Fr atrial cannula is used (see Fig. 56-1). Moving the pump above or below this level can affect flow. Adjusting the height of the blood pump alters filling of the blood chambers. It is important to allow 2 minutes for the system to adjust before making additional changes.
The vertically aligned pneumatic blood pumps are adjusted to optimize flow. The blood flow from the patient to the blood pump depends on the console used. The BVS 5000t (transport) console and the AB 5000 console allow for vacuum-assisted filling of the chamber. Filling of the pumps depends solely on gravity when the BVS 5000 or BVS 5000i (high-flow) consoles are used. The top of the blood pump should be between 0 and 10 inches below the level of the patient’s atria when a 42 Fr atrial cannula is used and 4 to 14 inches when a 32 Fr or 36 Fr atrial cannula is used (see Fig. 56-1). Moving the pump above or below this level can affect flow. Adjusting the height of the blood pump alters filling of the blood chambers. It is important to allow 2 minutes for the system to adjust before making additional changes.
 Outflow from the BVS is used in place of cardiac output for calculations such as systemic vascular resistance, pulmonary vascular resistance, and cardiac index.
Outflow from the BVS is used in place of cardiac output for calculations such as systemic vascular resistance, pulmonary vascular resistance, and cardiac index.
 External heat is not applied to the blood pump, tubing, or cannulas.
External heat is not applied to the blood pump, tubing, or cannulas.
 ABIOMED tubing insulators are used to retain heat in the tubing.
ABIOMED tubing insulators are used to retain heat in the tubing.
 The AB 5000 ventricle is a pulsatile, pneumatically driven blood pump approved for short-term (less than 3 weeks) support to allow time for myocardial recovery. It can provide support for one or both ventricles and must be used in conjunction with the AB 5000 circulatory support system console. The ventricle holds approximately 100 mL of blood. Cannulas exit the skin, and the ventricle lies on the patient’s abdomen. Filling of the ventricle is facilitated with a vacuum within the console and is not affected by height. The ventricle is made partially of aluminum and plastic and has inflow and outflow valves to ensure unidirectional blood flow.
The AB 5000 ventricle is a pulsatile, pneumatically driven blood pump approved for short-term (less than 3 weeks) support to allow time for myocardial recovery. It can provide support for one or both ventricles and must be used in conjunction with the AB 5000 circulatory support system console. The ventricle holds approximately 100 mL of blood. Cannulas exit the skin, and the ventricle lies on the patient’s abdomen. Filling of the ventricle is facilitated with a vacuum within the console and is not affected by height. The ventricle is made partially of aluminum and plastic and has inflow and outflow valves to ensure unidirectional blood flow.
 The following items must never come into contact with the ventricle because they could damage the plastic within the ventricle: ketones, such as acetone; aromatic hydrocarbons, such as gasoline; halogenated hydrocarbon–based anesthetic agents; other hydrocarbonated hydrocarbons, such as chloroform; and highly alkaline chemicals, such as sodium hydroxide.
The following items must never come into contact with the ventricle because they could damage the plastic within the ventricle: ketones, such as acetone; aromatic hydrocarbons, such as gasoline; halogenated hydrocarbon–based anesthetic agents; other hydrocarbonated hydrocarbons, such as chloroform; and highly alkaline chemicals, such as sodium hydroxide.
 Anticoagulation therapy with heparin initially followed by warfarin (Coumadin) is necessary.
Anticoagulation therapy with heparin initially followed by warfarin (Coumadin) is necessary.
 Thoratec Corporation offers an implantable VAD for left ventricular assistance, the HeartMate XVE LVAD.
Thoratec Corporation offers an implantable VAD for left ventricular assistance, the HeartMate XVE LVAD.
 This VAD is used for long-term support.
This VAD is used for long-term support.
 The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
The LVAD may be implanted intra-abdominally or preperitoneally in a rectus muscle pocket.
 The HeartMate XVE LVAD is made of titanium, holds 83 mL of blood, and weighs approximately 3 lb. Blood flows through a small tube placed in the left ventricular apex through a porcine inflow valve into the pump. When the pump fills with blood, a sensor inside the device starts the electric motor. Blood is pumped through a second porcine outflow valve through a graft into the aorta. The LVAD is placed in the left upper quadrant of the abdomen.
The HeartMate XVE LVAD is made of titanium, holds 83 mL of blood, and weighs approximately 3 lb. Blood flows through a small tube placed in the left ventricular apex through a porcine inflow valve into the pump. When the pump fills with blood, a sensor inside the device starts the electric motor. Blood is pumped through a second porcine outflow valve through a graft into the aorta. The LVAD is placed in the left upper quadrant of the abdomen.
 A driveline is passed underneath the skin and exits the right upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit) and vents the electric motor that is within the LVAD. The LVAD can run on the fixed rate mode (set rate) or on the automatic mode, which responds to changes in preload. On the automatic mode, the pump rate varies between 50 and 120 beats/min to meet the physiologic needs of the patients. Anticoagulation therapy with heparin or warfarin (Coumadin) is not necessary with this LVAD. Most patients receive aspirin, 81 mg or 325 mg daily, however.
A driveline is passed underneath the skin and exits the right upper quadrant of the abdomen. The driveline connects the LVAD to a controller and a power source (batteries or a power base unit) and vents the electric motor that is within the LVAD. The LVAD can run on the fixed rate mode (set rate) or on the automatic mode, which responds to changes in preload. On the automatic mode, the pump rate varies between 50 and 120 beats/min to meet the physiologic needs of the patients. Anticoagulation therapy with heparin or warfarin (Coumadin) is not necessary with this LVAD. Most patients receive aspirin, 81 mg or 325 mg daily, however.
 The device can be operated with the HeartMate IP console if the electric motor fails. Due to the increased risk of thrombosis, this mode is only used for short duration until the device can be replaced. The patient will also be anticoagulated with heparin or warfarin during this mode of operation.
The device can be operated with the HeartMate IP console if the electric motor fails. Due to the increased risk of thrombosis, this mode is only used for short duration until the device can be replaced. The patient will also be anticoagulated with heparin or warfarin during this mode of operation.
 The Thoratec Paracorporeal VAD (PVAD) is made of polyurethane and is pneumatically driven. A flexible polyurethane diaphragm divides the blood chamber. An influx of pressurized air (through the pneumatic tubing and into the VAD) drives the flexible diaphragm against the blood chamber, pushing blood from the VAD to the patient, and controls the duration of systole. Mechanical valves provide unidirectional blood flow. When the pneumatic drive is used, the pump can only be run in a fixed mode (asynchronous) or auto mode (volume) for right, left, or biventricular support
The Thoratec Paracorporeal VAD (PVAD) is made of polyurethane and is pneumatically driven. A flexible polyurethane diaphragm divides the blood chamber. An influx of pressurized air (through the pneumatic tubing and into the VAD) drives the flexible diaphragm against the blood chamber, pushing blood from the VAD to the patient, and controls the duration of systole. Mechanical valves provide unidirectional blood flow. When the pneumatic drive is used, the pump can only be run in a fixed mode (asynchronous) or auto mode (volume) for right, left, or biventricular support
 The Thoratec PVAD is approved for use as a bridge to transplant and post-cardiotomy recovery.
The Thoratec PVAD is approved for use as a bridge to transplant and post-cardiotomy recovery.
 The Thoratec PVAD is connected via the driveline and a cable to the dual-drive console (DDC), which controls pump function. The console is plugged into an electrical outlet when the patient is not ambulating. This VAD is primarily for short-term and intermediate use. The VADs may also be connected to the smaller TLC-II driver giving the patient 2 hours of battery life and a smaller 20 pound driver to push rather than the 500-pound dual-drive hospital driver (DDC).
The Thoratec PVAD is connected via the driveline and a cable to the dual-drive console (DDC), which controls pump function. The console is plugged into an electrical outlet when the patient is not ambulating. This VAD is primarily for short-term and intermediate use. The VADs may also be connected to the smaller TLC-II driver giving the patient 2 hours of battery life and a smaller 20 pound driver to push rather than the 500-pound dual-drive hospital driver (DDC).
 The Thoratec PVAD may be used in smaller patients.
The Thoratec PVAD may be used in smaller patients.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


56: Ventricular Assist Devices




