The inadequate relief of postoperative pain has adverse physiologic effects that can contribute to significant morbidity and mortality, resulting in the delay of patient recovery and return to daily activities.
 The pain pathway is not “hardwired” and nociceptive input is not passively transmitted from the periphery to the brain. Tissue injury tends to fuel neuroplastic changes within the nervous system, which results in both peripheral and central sensitization.
The pain pathway is not “hardwired” and nociceptive input is not passively transmitted from the periphery to the brain. Tissue injury tends to fuel neuroplastic changes within the nervous system, which results in both peripheral and central sensitization.
 In order for preventive analgesia to be successful, three critical principles must be adhered to: (1) The depth of analgesia must be adequate enough to block all nociceptive input during surgery, (2) the analgesic technique must be extensive enough to include the entire surgical field, and (3) the duration of analgesia must include both the surgical and postsurgical periods.
In order for preventive analgesia to be successful, three critical principles must be adhered to: (1) The depth of analgesia must be adequate enough to block all nociceptive input during surgery, (2) the analgesic technique must be extensive enough to include the entire surgical field, and (3) the duration of analgesia must include both the surgical and postsurgical periods.
 The various opioid analgesics available today have distinct pharmacologic differences that we can credit to their intricate interaction with the three main opioid receptors mu, delta, and kappa. The opioid receptors are members of a G protein–coupled (guanosine triphosphate regulatory proteins) receptor family, which signals via a second messenger such as cyclic adenosine monophosphate or an ion channel.
The various opioid analgesics available today have distinct pharmacologic differences that we can credit to their intricate interaction with the three main opioid receptors mu, delta, and kappa. The opioid receptors are members of a G protein–coupled (guanosine triphosphate regulatory proteins) receptor family, which signals via a second messenger such as cyclic adenosine monophosphate or an ion channel.
 The therapeutic benefit of nonsteroidal anti-inflammatory drugs is believed to be mediated through the inhibition of cyclooxygenase enzymes, types 1 and 2, which convert arachidonic acid to prostaglandins.
The therapeutic benefit of nonsteroidal anti-inflammatory drugs is believed to be mediated through the inhibition of cyclooxygenase enzymes, types 1 and 2, which convert arachidonic acid to prostaglandins.
 Short-term use of parecoxib and valdecoxib in patients following coronary artery bypass surgery is associated with an increased risk of thromboembolic events. The authors, therefore, do not recommend prescribing a cyclooxygenase 2 inhibitor for patients with a known history of coronary artery disease or cerebrovascular disease.
Short-term use of parecoxib and valdecoxib in patients following coronary artery bypass surgery is associated with an increased risk of thromboembolic events. The authors, therefore, do not recommend prescribing a cyclooxygenase 2 inhibitor for patients with a known history of coronary artery disease or cerebrovascular disease.
 The five variables associated with all modes of patient-controlled analgesia include (1) bolus dose, (2) incremental (demand) dose, (3) lockout interval, (4) background infusion rate, and (5) 1- and 4-hour limits. A typical patient-controlled analgesia regimen in an otherwise healthy adult would be an incremental dose of 1 to 2 mg of morphine with an 8- to 10-minute lockout. The authors do not recommend a background infusion of opioid in the opioid-naive patient.
The five variables associated with all modes of patient-controlled analgesia include (1) bolus dose, (2) incremental (demand) dose, (3) lockout interval, (4) background infusion rate, and (5) 1- and 4-hour limits. A typical patient-controlled analgesia regimen in an otherwise healthy adult would be an incremental dose of 1 to 2 mg of morphine with an 8- to 10-minute lockout. The authors do not recommend a background infusion of opioid in the opioid-naive patient.
 Epidural analgesia is a critical component of multimodal perioperative pain management and improved patient outcome. Meta-analyses investigating the efficacy of epidural analgesia found epidural analgesia to be superior to systemically administered opioids.
Epidural analgesia is a critical component of multimodal perioperative pain management and improved patient outcome. Meta-analyses investigating the efficacy of epidural analgesia found epidural analgesia to be superior to systemically administered opioids.
 Continuous peripheral nerve block has proven to be an effective technique for postoperative pain management, which is superior to opioid analgesia with fewer opioid-related side effects and rare neurologic and infectious complications.
Continuous peripheral nerve block has proven to be an effective technique for postoperative pain management, which is superior to opioid analgesia with fewer opioid-related side effects and rare neurologic and infectious complications.
 Should a perioperative nerve injury occur, it is incumbent on the physician to determine which combination of anesthetic, surgical, and patient risk factors are involved in any nerve injury and to not assume a priori that the regional anesthetic is the culprit.
Should a perioperative nerve injury occur, it is incumbent on the physician to determine which combination of anesthetic, surgical, and patient risk factors are involved in any nerve injury and to not assume a priori that the regional anesthetic is the culprit.
 The opioid-dependent patient is often identified just moments prior to surgery and the anesthesia team needs to be innovative. The anesthesiologist needs to be flexible enough to tailor an individual anesthetic that incorporates a multimodal approach, combining regional anesthesia with general anesthesia and nonopioid coanalgesics with opioid analgesics. Opioids remain the mainstay of perioperative pain management, and an adequate dose of opioid needs to be maintained to avoid precipitating withdrawal symptoms.
The opioid-dependent patient is often identified just moments prior to surgery and the anesthesia team needs to be innovative. The anesthesiologist needs to be flexible enough to tailor an individual anesthetic that incorporates a multimodal approach, combining regional anesthesia with general anesthesia and nonopioid coanalgesics with opioid analgesics. Opioids remain the mainstay of perioperative pain management, and an adequate dose of opioid needs to be maintained to avoid precipitating withdrawal symptoms.
 The key components to establishing a successful perioperative pain management service begins with an institutional commitment to support the service. The team must be built around a physician leader with training and experience in pain medicine. There must be other anesthesiologists available to support the service.
The key components to establishing a successful perioperative pain management service begins with an institutional commitment to support the service. The team must be built around a physician leader with training and experience in pain medicine. There must be other anesthesiologists available to support the service.
Multimedia
 Nociceptive Pathways
Nociceptive Pathways
 Pain Sensitization
Pain Sensitization
 Pain Processing
Pain Processing
 Universal Pain Assessment Tool
Universal Pain Assessment Tool
 Brachial Plexus
Brachial Plexus
 Interscalene Block
Interscalene Block
 Supraclavicular Block
Supraclavicular Block
 Femoral Nerve Block
Femoral Nerve Block
 Ultrasound Guided Saphenous Nerve Block
Ultrasound Guided Saphenous Nerve Block
 Ultrasound Guided Poplietal Nerve Block
Ultrasound Guided Poplietal Nerve Block
 TAP Block
TAP Block
Introduction
There are approximately 75 million surgical procedures performed each year in the United States and more than half are performed in the inpatient setting. Appropriate management of acute perioperative pain using multimodal or balanced analgesia is therefore crucial. In 1992 clinical practice guidelines were promulgated by the Agency for Health Care Policy and Research, which provided guidelines for physicians for the treatment of acute pain.1 Soon thereafter the American Society of Anesthesiologists developed treatment guidelines for acute postoperative pain, which have been updated and amended as of 2012.2 Despite significant advances in our knowledge and treatment of acute pain and dissemination of these guidelines, significant deficits continue to persist and the management of acute postoperative pain is still less than optimal.
 The inadequate relief of postoperative pain has adverse physiologic effects that can contribute to significant morbidity and mortality, resulting in the delay of patient recovery and return to daily activities.3 In addition, poor postoperative pain control contributes to patient dissatisfaction with the surgical experience and may have adverse psychological consequences.4 Poorly managed postoperative pain can also increase the incidence of persistent postoperative pain conditions. Because aggressive treatment of acute postoperative pain is considered to be so beneficial, the Joint Commission on Accreditation of Healthcare Organizations has declared that “pain is the fifth vital sign” and all institutions seeking accreditation from this group must develop pain management programs.
The inadequate relief of postoperative pain has adverse physiologic effects that can contribute to significant morbidity and mortality, resulting in the delay of patient recovery and return to daily activities.3 In addition, poor postoperative pain control contributes to patient dissatisfaction with the surgical experience and may have adverse psychological consequences.4 Poorly managed postoperative pain can also increase the incidence of persistent postoperative pain conditions. Because aggressive treatment of acute postoperative pain is considered to be so beneficial, the Joint Commission on Accreditation of Healthcare Organizations has declared that “pain is the fifth vital sign” and all institutions seeking accreditation from this group must develop pain management programs.
ACUTE PAIN DEFINED
Acute pain has been defined as “the normal, predicted, physiologic response to an adverse chemical, thermal, or mechanical stimulus.”5 Generally, acute pain resolves within 1 month. However, poorly managed acute pain that might occur following surgery can produce pathophysiologic processes in both the peripheral and central nervous systems that have the potential to produce chronicity.4 Acute pain-induced change in the central nervous system is known as neuronal plasticity. This can result in sensitization of the nervous system resulting in allodynia and hyperalgesia. Surgical procedures that can be associated with chronic painful conditions include amputation of a limb, lateral thoracotomy, inguinal herniorrhaphy, abdominal hysterectomy, saphenous vein stripping, open cholecystectomy, nephrectomy, and mastectomy.4
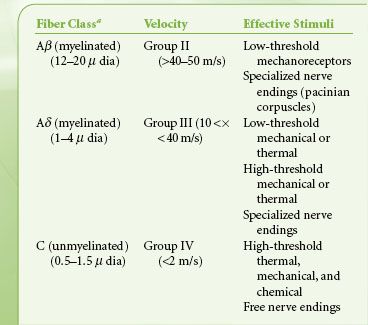
ANATOMY OF ACUTE PAIN
 The nociceptive pathway is an afferent (Fig. 56-1) three-neuron dual ascending (e.g., anterolateral and dorsal column medial lemniscal pathways) system, with descending modulation (Fig. 56-2) from the cortex, thalamus, and brainstem.6 Nociceptors are free nerve endings located in skin, muscle, bone, and connective tissue with cell bodies located in the dorsal root ganglia. The first-order neurons that make up the dual ascending system have their origins in the periphery as A delta and polymodal C fibers (Table 56-1). A delta fibers transmit “first pain,” which is described as sharp or stinging in nature and is well localized. Polymodal C fibers transmit “second pain,” which is more diffuse in nature and is associated with the affective and motivational aspects of pain. First-order neurons synapse on second-order neurons in the dorsal horn primarily within laminas I, II, and V, where they release excitatory amino acids and neuropeptides (Figs. 56-3 and 56-4). Some fibers can ascend or descend in Lissauer’s tract prior to terminating on neurons that project to higher centers. Second-order neurons consist of nociceptive-specific and wide dynamic-range (WDR) neurons. Nociceptive-specific neurons are located primarily in lamina I, respond only to noxious stimuli, and are thought to be involved in the sensory-discriminative aspects of pain. WDR neurons are predominately located in laminae IV, V, and VI, respond to both nonnoxious and noxious input, and are involved with the affective– motivational component of pain. Axons of both nociceptive-specific and WDR neurons ascend the spinal cord via the dorsal column-medial lemniscus and the anterior lateral spinothalamic tract to synapse on third-order neurons in the contralateral thalamus, which then project to the somatosensory cortex where nociceptive input is perceived as pain (Fig. 56-1).
The nociceptive pathway is an afferent (Fig. 56-1) three-neuron dual ascending (e.g., anterolateral and dorsal column medial lemniscal pathways) system, with descending modulation (Fig. 56-2) from the cortex, thalamus, and brainstem.6 Nociceptors are free nerve endings located in skin, muscle, bone, and connective tissue with cell bodies located in the dorsal root ganglia. The first-order neurons that make up the dual ascending system have their origins in the periphery as A delta and polymodal C fibers (Table 56-1). A delta fibers transmit “first pain,” which is described as sharp or stinging in nature and is well localized. Polymodal C fibers transmit “second pain,” which is more diffuse in nature and is associated with the affective and motivational aspects of pain. First-order neurons synapse on second-order neurons in the dorsal horn primarily within laminas I, II, and V, where they release excitatory amino acids and neuropeptides (Figs. 56-3 and 56-4). Some fibers can ascend or descend in Lissauer’s tract prior to terminating on neurons that project to higher centers. Second-order neurons consist of nociceptive-specific and wide dynamic-range (WDR) neurons. Nociceptive-specific neurons are located primarily in lamina I, respond only to noxious stimuli, and are thought to be involved in the sensory-discriminative aspects of pain. WDR neurons are predominately located in laminae IV, V, and VI, respond to both nonnoxious and noxious input, and are involved with the affective– motivational component of pain. Axons of both nociceptive-specific and WDR neurons ascend the spinal cord via the dorsal column-medial lemniscus and the anterior lateral spinothalamic tract to synapse on third-order neurons in the contralateral thalamus, which then project to the somatosensory cortex where nociceptive input is perceived as pain (Fig. 56-1).
FIGURE 56-1. Afferent nociceptive pathway.
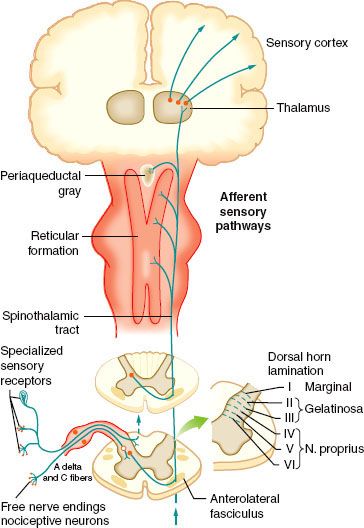
FIGURE 56-2. Efferent pathways involved in nociceptive regulation.
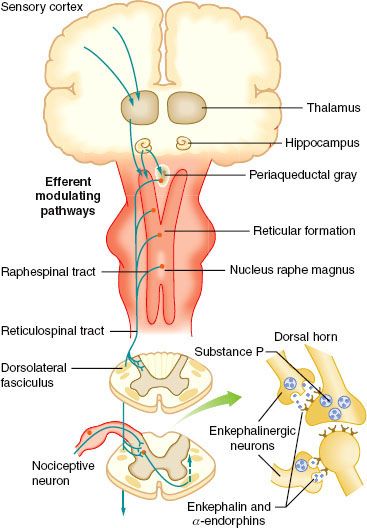
FIGURE 56-3. Schematic on the right showing the Rexed lamination and the approximate organization of the approach of the afferent to the spinal cord as they enter at the dorsal root entry zone and then penetrate into the dorsal horn to terminate in laminae I and II (A/C) or penetrate more deeply to loop upward to terminate as high as the dorsum of lamina III (Aβ). Inset on the left shows histologic appearance of the left dorsal quadrant, and large, myelinated axons. (From: Warfield CA, Bajwa ZH, eds. Principles and Practice of Pain Medicine. 2nd ed. New York, NY: McGraw Hill Medical Publishing Division; 2004.)
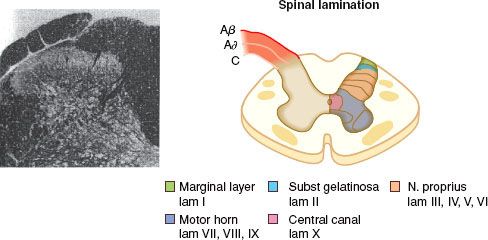
FIGURE 56-4. Schematic summarizing the organization of dorsal horn systems that contribute to the processing of nociceptive information. (1) Primary afferent C fibers release peptide (e.g., substance P [sP], calcitonin gene–related peptide [CGRP], and so on) and excitatory amino acid (glutamate) products. Small dorsal root ganglion (DRG) cells, as well as some postsynaptic elements contain nitric oxide synthase (NOS) and are able, upon depolarization, to release NO (nitric oxide). (2) Peptides and excitatory amino acids evoke excitation in second-order neurons. For glutamate, direct monosynaptic excitation is mediated by non–N-methyl-D-aspartate (NMDA) receptors (i.e., acute primary afferent excitation of WDR neurons is not mediated by the NMDA or neurokinin 1 (NK-1) receptor). (3) Interneurons excited by afferent barrage induce excitation in second-order neurons via an NMDA receptor. This leads to a marked increase in intracellular Ca2+ and the activation of kinases and phosphorylating enzymes. Prostaglandins (PGs) generated by cyclooxygenase-2 (COX-2) and NO by NOS are formed and released. These agents diffuse extracellulary and facilitate transmitter release (retrograde transmission) from primary and nonprimary afferent terminals, either by a direct cellular action (e.g., NO) or by an interaction with a specific class of receptors (e.g., EP receptors for prostanoids). (4) Nonneuronal sources of prostaglandins may include activated astrocytes and microglia that are stimulated by circulating cytokines, which are released secondary to peripheral nerve injury and inflammation. Terminal excitability can be altered by activation of a variety of receptors located on the sensory terminal, including those for, μ, δ, and κ opioids. (From: Warfield CA, Bajwa ZH, eds. Principles and Practice of Pain Medicine. 2nd ed. New York, NY: McGraw Hill Medical Publishing Division; 2004.)
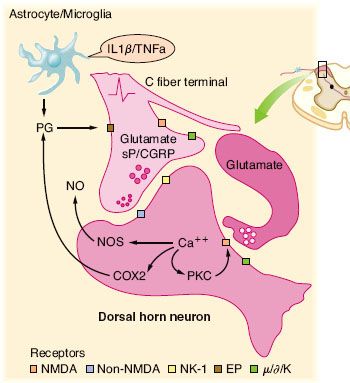
TABLE 56-1. PRIMARY AFFERENT NERVES
Pain Processing
 A key development in our understanding of pain processing is that the pain pathway is not “hardwired” and nociceptive input is not passively transmitted from the periphery to the brain.7 Tissue injury tends to fuel neuroplastic changes within the nervous system, which results in both peripheral and central sensitization.
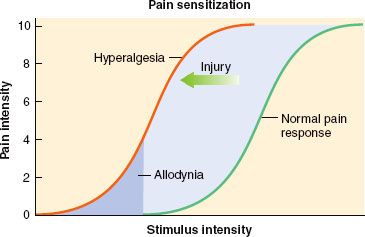
A key development in our understanding of pain processing is that the pain pathway is not “hardwired” and nociceptive input is not passively transmitted from the periphery to the brain.7 Tissue injury tends to fuel neuroplastic changes within the nervous system, which results in both peripheral and central sensitization. Clinically this can manifest as hyperalgesia, which is defined as an exaggerated pain response to a normally painful stimulus, and allodynia, which is defined as a painful response to a typically nonpainful stimulus7 (Fig. 56-5).
Clinically this can manifest as hyperalgesia, which is defined as an exaggerated pain response to a normally painful stimulus, and allodynia, which is defined as a painful response to a typically nonpainful stimulus7 (Fig. 56-5).
FIGURE 56-5. Pain sensitization. (Obtained with permission from: Dave Klemm, http:www.georgetown.edu/dml/facs/graphics/index.htm.)
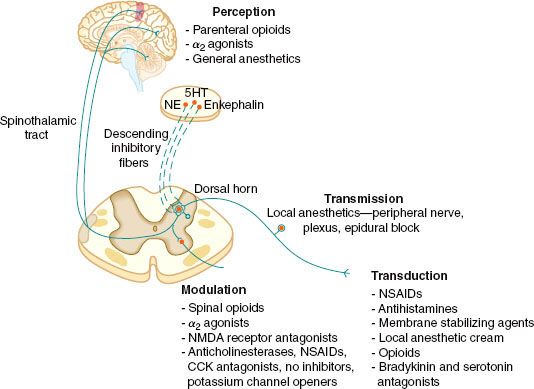
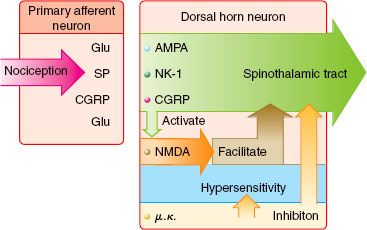
 The four elements of pain processing include (1) transduction, (2) transmission, (3) modulation, and (4) perception (Fig. 56-6). Transduction is the event whereby noxious thermal, chemical, or mechanical stimuli are converted into an action potential. Transmission occurs when the action potential is conducted through the nervous system via the first-, second-, and third-order neurons, which have cell bodies located in the dorsal root ganglion, dorsal horn, and thalamus, respectively. Modulation of pain transmission involves altering afferent neural transmission along the pain pathway. The dorsal horn of the spinal cord is the most common site for modulation of the pain pathway, and modulation can involve either inhibition or augmentation of the pain signals.6 Examples of inhibitory spinal modulation include (1) release of inhibitory neurotransmitters such as γ-amino butyric acid (GABA) and glycine by intrinsic spinal neurons, and (2) activation of descending efferent neuronal pathways from the motor cortex, hypothalamus, periaqueductal gray matter, and the nucleus raphe magnus, which results in the release of norepinephrine, serotonin, and endorphins in the dorsal horn. Spinal modulation, which results in augmentation of pain pathways, is manifested as central sensitization, which is a consequence of neuronal plasticity. The phenomenon of “wind-up” is a specific example of central plasticity that results from repetitive C-fiber stimulation of WDR neurons in the dorsal horn. Perception of pain is the final common pathway, which results from the integration of painful input into the somatosensory and limbic cortices. Generally speaking, traditional analgesic therapies have only targeted pain perception. A multimodal approach to pain therapy should target all four elements of the pain processing pathway.
The four elements of pain processing include (1) transduction, (2) transmission, (3) modulation, and (4) perception (Fig. 56-6). Transduction is the event whereby noxious thermal, chemical, or mechanical stimuli are converted into an action potential. Transmission occurs when the action potential is conducted through the nervous system via the first-, second-, and third-order neurons, which have cell bodies located in the dorsal root ganglion, dorsal horn, and thalamus, respectively. Modulation of pain transmission involves altering afferent neural transmission along the pain pathway. The dorsal horn of the spinal cord is the most common site for modulation of the pain pathway, and modulation can involve either inhibition or augmentation of the pain signals.6 Examples of inhibitory spinal modulation include (1) release of inhibitory neurotransmitters such as γ-amino butyric acid (GABA) and glycine by intrinsic spinal neurons, and (2) activation of descending efferent neuronal pathways from the motor cortex, hypothalamus, periaqueductal gray matter, and the nucleus raphe magnus, which results in the release of norepinephrine, serotonin, and endorphins in the dorsal horn. Spinal modulation, which results in augmentation of pain pathways, is manifested as central sensitization, which is a consequence of neuronal plasticity. The phenomenon of “wind-up” is a specific example of central plasticity that results from repetitive C-fiber stimulation of WDR neurons in the dorsal horn. Perception of pain is the final common pathway, which results from the integration of painful input into the somatosensory and limbic cortices. Generally speaking, traditional analgesic therapies have only targeted pain perception. A multimodal approach to pain therapy should target all four elements of the pain processing pathway.
FIGURE 56-6. The four elements of pain processing: Transduction, transmission, modulation, and perception. 5HT, serotonin; NE, norepinephrine; NMDA, N-methyl-D-asparate; NSAIDs, nonsteroidal anti-inflammatory drugs; CCK, cholecystokin; NO, nitric oxide.
CHEMICAL MEDIATORS OF TRANSDUCTION AND TRANSMISSION
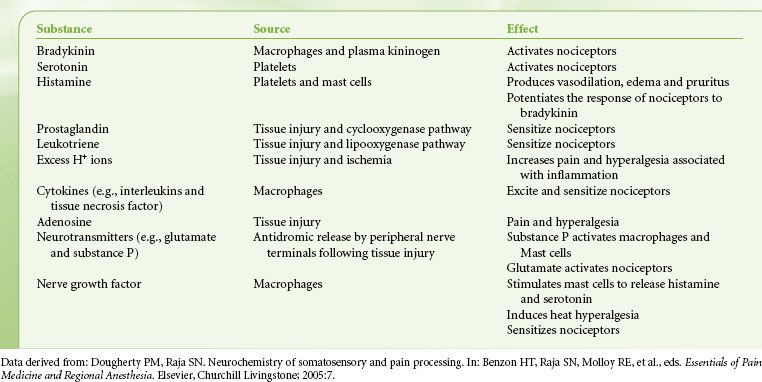
Tissue damage following surgical procedures leads to the activation of small nociceptive nerve endings and local inflammatory cells (e.g., macrophages, mast cells, lymphocytes, and platelets) in the periphery. Antidromic release of substance P and glutamate from small nociceptive afferents results in vasodilation, extravasation of plasma proteins, and stimulation of inflammatory cells to release numerous algogenic substances (Table 56-2 and Fig. 56-7). This chemical milieu will both directly produce pain transduction via nociceptor stimulation as well as facilitate pain transduction by increasing the excitability of nociceptors. Peripheral sensitization of polymodal C fibers and high-threshold mechanoreceptors by these chemicals leads to primary hyperalgesia, which by definition is an exaggerated response to pain at the site of injury.
FIGURE 56-7. Schematic of the neurochemistry of somatosensory processing at peripheral sensory nerve endings. (From: Benzon HT, Raja SN, Molloy RE, et al., eds. Essentials of Pain Medicine and Regional Anesthesia. 2nd ed. Elsevier, Churchill Livingstone; 2005:8.)
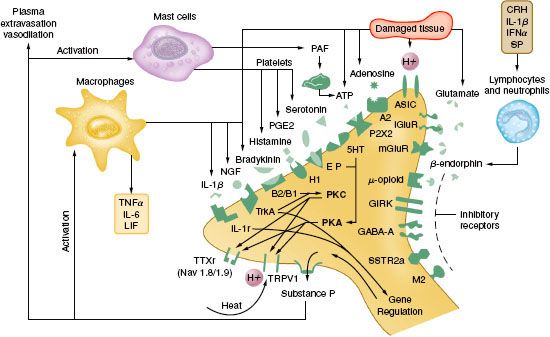
TABLE 56-2. ALGOGENIC SUBSTANCES
As is the case in the periphery, the dorsal horn of the spinal cord contains numerous transmitters and receptors involved in pain processing. Three classes of transmitter compounds integral to pain transmission include (1) the excitatory amino acids glutamate and aspartate, (2) the excitatory neuropeptides substance P and neurokinin A, and (3) the inhibitory amino acids glycine and GABA. The various pain receptors include (1) the NMDA (N-methyl-D-aspartate), (2) the AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-proprionic acid), (3) the kainate, and (4) the metabotropic (Fig. 56-8).
FIGURE 56-8. Schematic representation of peripheral and spinal mechanism involved in neuroplasticity. Primary hyperalgesia results from tissue release of toxic substances. These toxic substances spread to adjacent tissues, prolonging the hyperalgesic state (secondary hyperalgesia). As C fiber terminals increase in frequency of release of neurotransmitters, such as glutamate, substance P, tachykinins, brain-derived neurotrophic factor, and calcitonin gene–related peptide, the effects of these neurotransmitters are summated, resulting in prolonged depolarizations of second-order neurons (“wind-up”). Function changes at the second-order neuron occur as a result of neurotransmitter binding to postsynaptic receptors, which results in activity-dependent plasticity of the spinal cord. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; NK, neurokinin; NMDA, N-methyl-D-asparate.
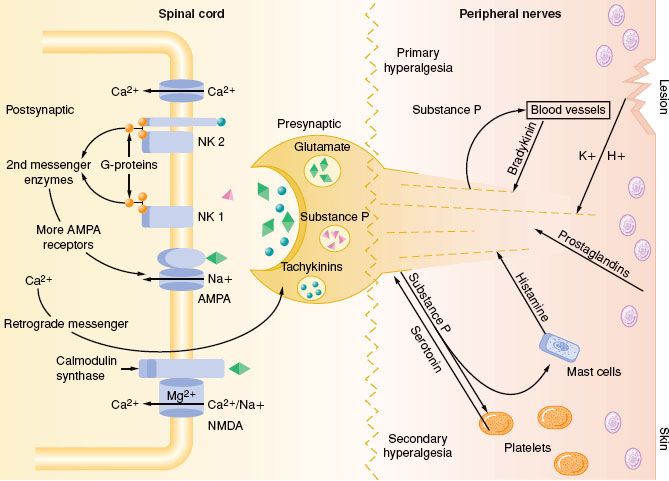
FIGURE 56-9. Primary nociceptive transmission in the spinal cord. Primary afferent nociceptive input is transmitted via α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), neurokinin-1 (NK1), and calcitonin gene–related peptide (CGRP) synapses, whose signals work their way to the thalamus. Glutaminergic (N-methyl-D-asparate [NMDA]) synapses do not participate significantly in primary nociceptive transmission, but instead play a crucial role in spinal sensitization. Accordingly, even after complete NMDA blockade in the spinal cord, primary afferent nociceptive information is transmitted to the thalamus. NMDA antagonists thus have an antihyperalgesic rather than an analgesic effect in the spinal cord. Glu, glutamate; SP, substance P. (Reprinted from: International Association for the Study of Pain: Pain Control Updates. IASP Newletter 2005;13(2):3, with permission.)
The AMPA and kainate receptors, which are sodium channel dependent, are essential for fast synaptic afferent input. On the other hand, the NMDA receptor, which is calcium channel dependent, is only activated following prolonged depolarization of the cell membrane. Release of substance P into the spinal cord will remove the magnesium block on the channel of the NMDA receptor giving glutamate free access to the NMDA receptor. Repetitive C-fiber stimulation of WDR neurons in the dorsal horn at intervals of 0.5 to 1 Hz can precipitate the occurrence of wind-up and central sensitization (Fig. 56-9). This leads to secondary hyperalgesia, which, by definition, is an increased pain response evoked by stimuli outside the area of injury.
THE SURGICAL STRESS RESPONSE
Although similar, postoperative pain and the surgical stress response are not the same. Surgical stress causes release of cytokines (e.g., interleukin-1, interleukin-6, and tumor necrosis factor-α) and precipitates adverse neuroendocrine and sympathoadrenal responses, resulting in detrimental physiologic responses, particularly in high-risk patients.4
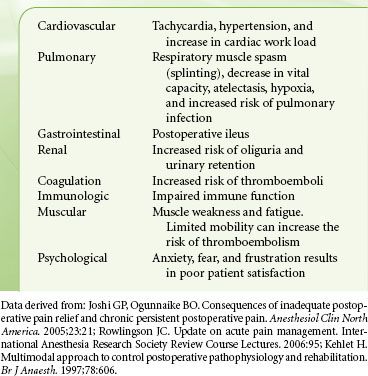
The increased secretion of the catabolic hormones such as cortisol, glucagon, growth hormone, and catecholamines and the decreased secretion of the anabolic hormones such as insulin and testosterone, characterize the neuroendocrine response. The end result of this is hyperglycemia and a negative nitrogen balance, the consequences of which include poor wound healing, muscle wasting, fatigue, and impaired immunocompetency.
The sympathoadrenal response has detrimental effects on numerous organ systems; these are listed in Table 56-3.8
TABLE 56-3. CONSEQUENCES OF POORLY MANAGED ACUTE PAIN
PREVENTIVE ANALGESIA
Preventive analgesia includes any antinociceptive regimen delivered at any time during the perioperative period that will attenuate pain-induced sensitization. The term “preventive analgesia” replaces the older terminology “preemptive analgesia”, which is defined as an analgesic regimen that is administered prior to surgical incision and is more effective at pain relief than the same regimen administered after surgery. Although use of the term preemptive analgesia has been popular in the past, evidence of its clinical benefit in humans has been mixed and the term should be considered obsolete. The goal of preventive analgesia is to block the development of sustained pain. Theoretically, this occurs by preventing NMDA receptor activation in the dorsal horn that is associated with wind-up, facilitation, central sensitization expansion of receptive fields, and long-term potentiation, all of which can lead to a chronic pain state.7  In order for preventive analgesia to be successful, three critical principles must be adhered to: (1) The depth of analgesia must be adequate enough to block all nociceptive input during surgery, (2) the analgesic technique must be extensive enough to include the entire surgical field, and (3) the duration of analgesia must include both the surgical and postsurgical periods. Patients with pre-existing chronic pain may not respond as well to these techniques because of pre-existing sensitization of the nervous system.
In order for preventive analgesia to be successful, three critical principles must be adhered to: (1) The depth of analgesia must be adequate enough to block all nociceptive input during surgery, (2) the analgesic technique must be extensive enough to include the entire surgical field, and (3) the duration of analgesia must include both the surgical and postsurgical periods. Patients with pre-existing chronic pain may not respond as well to these techniques because of pre-existing sensitization of the nervous system.
STRATEGIES FOR ACUTE PAIN MANAGEMENT
The majority of postoperative pain is nociceptive in character, but there are a small percentage of patients who can experience neuropathic pain postoperatively. It is critical to recognize this fact because patients with neuropathic pain are at increased risk of progressing to a chronic pain state. Neuropathic pain is a result of accidental nerve injury secondary to cutting, traction compression, or entrapment.4 Clinical features may include continuous burning, paroxysmal shooting, or electric pain with associated allodynia, hyperalgesia, and dysesthesias. There can be a delay in the onset of the pain, and it can follow a nondermatomal distribution. Surgical procedures that are a relatively high risk for neuropathic pain include limb amputations, breast surgery, gallbladder surgery, thoracic surgery, and inguinal hernia repair.4 Nociceptive pain responds best to opioids, nonsteroidal anti-inflammatory drugs (NSAIDs), para-aminophenol agents, and regional anesthesia techniques.10 Neuropathic pain, on the other hand, may benefit from the addition of the nonopioid analgesic adjuvants such as the NMDA receptor antagonists, α2-agonists, and the α2–δ subunit calcium channel ligands, which will be discussed in detail.
Strategies for acute pain management should also consider the sex of the patient as sex differences appear to exist for pain perception as well as response to opioid analgesics. Evidence suggests that women experience more pain following surgery than men, and therefore require more morphine to achieve a similar level of pain relief.11
ASSESSMENT OF ACUTE PAIN
The need for assessment of the patient in pain is illustrated by the postoperative patient who is said to be relatively pain-free, but who, on inspection, is lying almost completely still in bed. Too often, such a patient has had a recent cursory evaluation that included the traditional verbal analog score (VAS) 0 to 10 scale (“on a scale of 0 to 10, with 0 being no pain and 10 being the worst pain you can imagine, how much pain are you in” from which the patient reported a low VAS score of 1/10) (Fig. 56-10).  The treating team took that as reassuring information and moved along. No one asked the patient about pain with movement, breathing, moving bowels, and so forth, all potentially important functional goals for the postoperative course that may be undermined by untreated pain.
The treating team took that as reassuring information and moved along. No one asked the patient about pain with movement, breathing, moving bowels, and so forth, all potentially important functional goals for the postoperative course that may be undermined by untreated pain.
FIGURE 56-10. Linear verbal analog score and faces pain assessment tool.
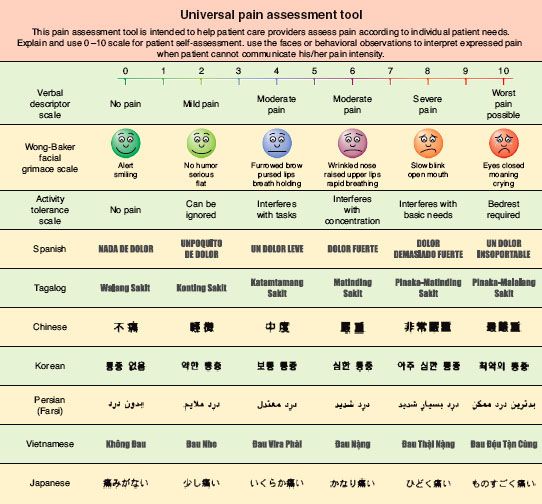
A variety of well-studied pain measurement scales exist that can be helpful yet are not definitive. Unidimensional instruments such as the familiar numerical pain scale already mentioned, the visual analog scale, and the “faces” (Fig. 56-10) pain rating scale can provide some degree of guidance about a patient’s experience of pain, but all of these are completely subjective and are open to wide variation between subjects and within subjects at different times.
Multidimensional instruments, such as the McGill Pain Questionnaire or the Brief Pain Inventory provide a broader picture of a patient’s experience, but are usually more cumbersome to administer and, in the end, suffer the same limitations as all other attempts to measure pain. A number of tools to assess cancer-related and noncancer chronic pain have been advanced and validated.12 Most of these focus on persistent background pain and do not help identify intermittent or breakthrough pain. Several assessment scales specifically address breakthrough or episodic pain. The Breakthrough Pain Questionnaire was introduced by Portenoy and Hagen to assess breakthrough pain in cancer patients, and has also been studied in patients with acute noncancer pain, for which it can offer a picture of both breakthrough and background pain states.13
TABLE 56-4. THREE CLASSES OF ACUTE PAIN

Ultimately, we are left with a maxim first attributed to Dr. John Bonica, the father of pain medicine: “Pain is what a patient says it is.” The best way to begin assessing a patient’s pain is to ask about it and listen to the answers. Attempts to reduce the experience to finite details may lead to failure to ask the right questions, distance us from our patients, focus us away from the whole person, and potentially miss golden diagnostic clues that could lead to effective interventions.
Effective treatment of acute pain requires assessment as well as vigilant reassessment to determine if the primary goals are met, adversity has occurred, or changes are necessary. Acute pain may be viewed as breakthrough, intermittent, or background in nature (Table 56-4). The assessment process for each of these is relatively similar and will help to resolve the related condition into broad pathophysiologic groups such as cancer versus noncancer, and nociceptive versus neuropathic, or mixed pain states. Such an approach supports a rational process for developing a useful differential diagnosis and approaches. Table 56-5 lists the common features of pain that are usually reviewed during the assessment for acute pain. A thorough physical examination must also be performed with particular attention to the neurologic examination, which may offer clues to aberrant neural processing. Such neurologic findings may indicate nerve injury, alerting the astute clinician to a neuropathic rather than a nociceptive pain state that requires a different analgesic approach.14 A provocative physical examination may include examination of the affected areas with maneuvers that may provoke pain such as range of motion testing, walking, and cough. The benefits of provocative testing must outweigh the associated suffering incurred by the patient. Medical imaging is also a common part of the acute pain workup. Overemphasis on imaging data should, however, be avoided as this can potentially lead to misinterpretation of the patient’s underlying pain syndrome.
TABLE 56-5. FEATURES OF PAIN COMMONLY ADDRESSED DURING ASSESSMENT

OPIOID ANALGESICS
 Opioids are the mainstay for the treatment of acute postoperative pain, and morphine is the “gold-standard.” The various opioid analgesics available today have distinct pharmacologic differences that we can credit to their intricate interaction with the three main opioid receptors: mu, delta, and kappa. The opioid receptors are members of a G protein–coupled (guanosine triphosphate regulatory proteins) receptor family, which signals via a second messenger such as cyclic adenosine monophosphate or an ion channel. In the ascending pain pathway opioid receptors are located in three areas that include (1) the periphery, following inflammation; (2) the spinal cord dorsal horn; and (3) supraspinally in the brainstem, thalamus, and cortex. Mu opioid receptors are also found in the periaqueductal grey, the nucleus raphe magnus, and the rostral ventral medulla, which constitutes the descending inhibitory pain pathway. The three primary mechanisms of action for opioid analgesia at the level of the spinal cord, include (1) inhibition of calcium influx presynaptically, which results in depolarization of the cell membrane and decreased release of neurotransmitters and neuropeptides into the synaptic cleft; (2) enhancing potassium efflux from the cell postsynaptically, which results in hyperpolarization of the cell and a decrease in pain transmission, and (3) activation of a descending inhibitory pain circuit via inhibition of GABAergic transmission in the brainstem. Peripheral opioid receptors, which mediate analgesia, are located on primary afferent neurons. Activation of these receptors inhibits the release of pronociceptive and proinflammatory substances like substance P, which accounts for the analgesic and anti-inflammatory effects. The “broad-spectrum” opioid, methadone, has NMDA receptor antagonist properties and inhibits the reuptake of serotonin and norepinephrine, which may make it useful in the treatment of neuropathic pain.
Opioids are the mainstay for the treatment of acute postoperative pain, and morphine is the “gold-standard.” The various opioid analgesics available today have distinct pharmacologic differences that we can credit to their intricate interaction with the three main opioid receptors: mu, delta, and kappa. The opioid receptors are members of a G protein–coupled (guanosine triphosphate regulatory proteins) receptor family, which signals via a second messenger such as cyclic adenosine monophosphate or an ion channel. In the ascending pain pathway opioid receptors are located in three areas that include (1) the periphery, following inflammation; (2) the spinal cord dorsal horn; and (3) supraspinally in the brainstem, thalamus, and cortex. Mu opioid receptors are also found in the periaqueductal grey, the nucleus raphe magnus, and the rostral ventral medulla, which constitutes the descending inhibitory pain pathway. The three primary mechanisms of action for opioid analgesia at the level of the spinal cord, include (1) inhibition of calcium influx presynaptically, which results in depolarization of the cell membrane and decreased release of neurotransmitters and neuropeptides into the synaptic cleft; (2) enhancing potassium efflux from the cell postsynaptically, which results in hyperpolarization of the cell and a decrease in pain transmission, and (3) activation of a descending inhibitory pain circuit via inhibition of GABAergic transmission in the brainstem. Peripheral opioid receptors, which mediate analgesia, are located on primary afferent neurons. Activation of these receptors inhibits the release of pronociceptive and proinflammatory substances like substance P, which accounts for the analgesic and anti-inflammatory effects. The “broad-spectrum” opioid, methadone, has NMDA receptor antagonist properties and inhibits the reuptake of serotonin and norepinephrine, which may make it useful in the treatment of neuropathic pain.
There is great diversity in the available routes of administration of opioid analgesics. Table 56-6 is a list of relevant pharmacokinetic data. Table 56-7 offers equianalgesic dosing guidelines for the various opioids. The reader is referred to the section “Perioperative Pain Management of the Opioid-dependent Patient” for a complete discussion of incomplete cross-tolerance between the different opioids and dosing considerations.
TABLE 56-6. OPIOID ANALGESIC PHARMACOKINETICS
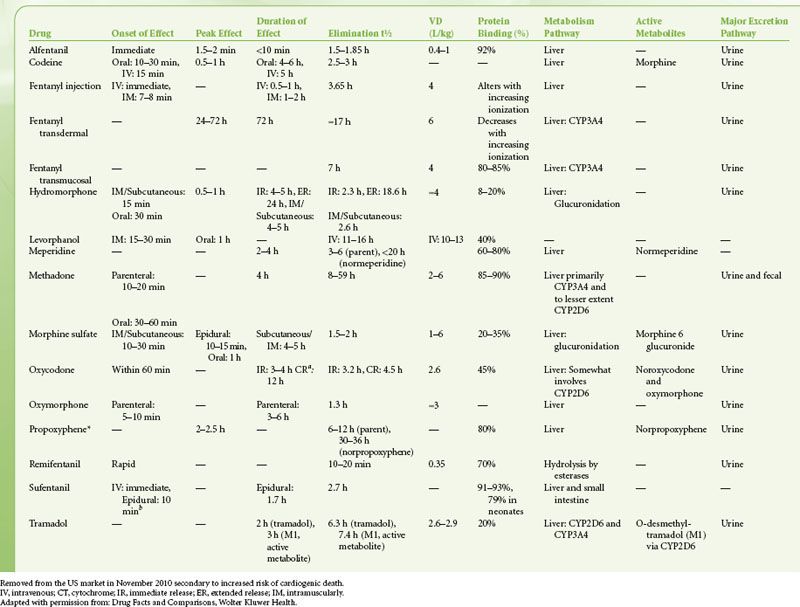
TABLE 56-7. OPIOID EQUIANALGESIC DOSING
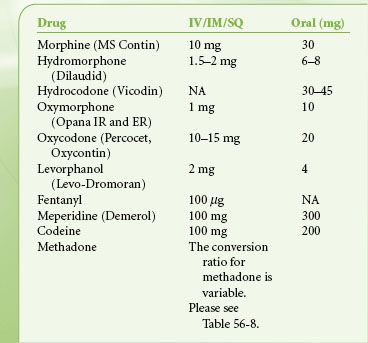
Common opioid-induced side effects include sedation, nausea and vomiting, respiratory depression, and constipation. Less common side effects include confusion, urinary retention, dizziness, and myoclonus. Tolerance rarely develops to the constipating effects of the opioids. Alvimopan, a peripherally acting mu receptor antagonist that has negligible systemic absorption, attenuates opioid-induced constipation and shortens postoperative ileus and length of hospital stay.15 Opioid-induced hyperalgesia (OIH) is a relatively rare phenomenon whereby patients who are receiving opioids suddenly and paradoxically become more sensitive to pain despite continued treatment with opioids. Evidence suggests that OIH is more likely to develop following high doses of phenanthrene opioids such as morphine.16 Changing the opioid to a phenyl piperidine derivative such as fentanyl may thwart OIH. There is also evidence that coadministration of an NMDA receptor antagonist can abolish opioid-induced tolerance and OIH.16 Finally, opioid analgesics have profound immunomodulatory effects, which include inhibition of cellular and humoral immune functions, depressed natural killer cell activity, promotion of angiogenesis and inhibition of apoptosis. Such effects can be beneficial or deleterious depending upon the clinical situation.17,18
Morphine is the prototype opioid and is the “gold standard” to which all other analgesics are compared. Although the plasma half-life of the drug is approximately 2 hours its analgesic duration of action is closer to 4 to 5 hours. Morphine undergoes hepatic glucuronidation to morphine-6-glucuronide and morphine-3-glucuronide, both of which are cleared by the kidney. Morphine-6-glucuronide is an active metabolite of morphine and is thought to be responsible for most of the analgesia associated with chronic dosing of the drug. Morphine-3-glucuronide, on the other hand, is considered to be devoid of analgesic activity. With chronic dosing these metabolites can accumulate and can be particularly problematic in patients with renal failure. Dosing adjustment is therefore necessary and monitoring of side effects is important. Morphine-6-glucuronide contributes to side effects such as drowsiness, nausea and vomiting, coma, and respiratory depression. Morphine-3-glucuronide, on the other hand, is thought to cause agitation, myoclonus, delirium, and hyperalgesia.
Hydromorphone is a semisynthetic opioid that has 4 to 6 times the potency of morphine. It is available for oral, rectal, parenteral, and neuraxial administration. Whereas the oral bioavailability of the drug is reported to be 20% to 50%, its bioavailability via the subcutaneous route is 78%, making it the ideal drug for long-term subcutaneous administration in the opioid-tolerant patient. Like morphine, hydromorphone is biotransformed in the liver. The active metabolites are dihydromorphine and dihydroisomorphine and the inactive metabolite is hydromorphone-3-glucuronide. Although hydromorphone has traditionally been the preferred opioid for patients with acute pain and impaired kidney function, evidence suggests that hydromorphone-3-glucuronide can accumulate in those with renal failure and may contribute to side effects such as neuroexcitation and cognitive impairment. Opioid-related side effects such as nausea, vomiting, sedation, cognitive impairment, and pruritus are reported to be less intense with hydromorphone vis-à-vis morphine. In fact, the incidence of pruritus following neuraxial administration of hydromorphone is reported to be approximately 5% versus the 11% to 77% range reported for neuraxial morphine.19
Fentanyl, a synthetic opioid chemically related to the phenylpiperidines, is a relatively selective mu receptor agonist, which is considered to be 80 times the potency of morphine following intravenous administration. It is extensively metabolized in the liver to norfentanyl and other inactive metabolites, which are excreted in the urine and bile. Fentanyl is therefore suitable for patients in renal failure. The drug is available for intravenous, subcutaneous, transdermal, transmucosal, and neuraxial administration. The transdermal administration of fentanyl using iontophoresis (Ionsys, Janssen-Cilag LTD) is a novel on-demand drug delivery system that does not require venous access.
Sufentanil, alfentanil, and remifentanil are analogs of fentanyl that have analgesic effects similar to those of morphine and the other mu receptor agonists. Sufentanil is approximately 1,000 times the potency of morphine and is primarily used in the operating room either intravenously or neuraxially.20 Like fentanyl, sufentanil is very lipophilic, and although their pharmacokinetic and pharmacodynamic profiles are similar, sufentanil has a smaller volume of distribution and shorter elimination half-life.20 The high intrinsic potency of sufentanil makes it an excellent choice for epidural analgesia in the opioid-dependent patient.21 Alfentanil is approximately 10 times the potency of morphine and, like sufentanil, is used primarily in the operating room either intravenously or neuraxially. Remifentanil is an ultra–short-acting synthetic opioid. The potency of the drug is approximately equal to that of fentanyl. Remifentanil is rapidly degraded by tissue and plasma esterases, which accounts for its incredibly short terminal elimination half-life of 10 to 20 minutes.20 Rapid clearance and lack of accumulation make this a very desirable opioid in the operative setting. One disadvantage, however, is that discontinuation of a remifentanil infusion results in rapid loss of analgesia. There is also evidence to suggest that remifentanil infusions may be associated with the development of opioid-induced hyperalgesia. Further studies are clearly needed to better define this phenomenon.
Meperidine, a phenylpiperidine, is a synthetic mu opioid receptor agonist with a short half-life. The drug is recommended for the short-term management of acute pain only and has absolutely no role in the management of chronic pain. The drug is biotransformed by the liver to normeperidine, a potentially neurotoxic metabolite, which has a 12- to 16-hour half-life. Repetitive dosing of meperidine can cause accumulation of normeperidine, which may precipitate tremulousness, myoclonus, and seizures. It is therefore recommended that the total daily intravenous dose in an otherwise healthy adult without renal or central nervous system disease should not exceed 600 mg/day and should not be administered for longer than 48 hours.22 We do not recommend administration of meperidine as an intravenous patient–controlled analgesia (PCA). The drug is contraindicated in patients receiving monoamine oxidase inhibitors as this may precipitate a syndrome characterized by muscle rigidity, hyperpyrexia, and seizures.
Methadone is a relatively inexpensive synthetic opioid considered to be a broad-spectrum opioid because it is a (1) mu receptor agonist, (2) NMDA antagonist, and (3) inhibitor of monoamine transmitter reuptake, making it potentially useful for the treatment of neuropathic pain. The drug is well absorbed from the gastrointestinal tract with a reported bioavailability of approximating 80%. The drug is extensively metabolized in the liver by the cytochrome P450 (CYP450) system to inactive metabolites, which are cleared in the bile and urine; unlike morphine, it is generally not necessary to adjust the dosage of methadone in patients with renal insufficiency. Methadone has an elimination half-life of 22 hours, and following a single dose the duration of analgesia is approximately 3 to 6 hours. With repetitive dosing, however, methadone can accumulate and slow tissue release into the blood stream can result in a long elimination half-life of up to 128 hours and duration of analgesia of 8 to 12 hours. This long half-life explains the potential risk for cumulative toxicity, and therefore the importance of monitoring for side effects such as excessive sedation and confusion following the initiation of an around-the-clock dosing regimen.
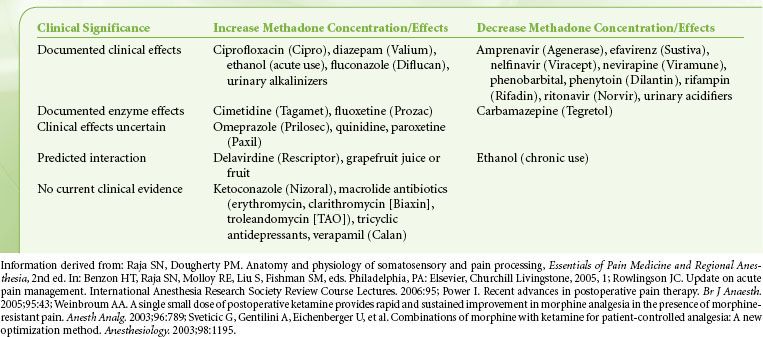
Finally, opioid rotation is a very useful technique to restore analgesic sensitivity in the highly tolerant patient, and methadone is a common choice for opioid rotation. Because cross-tolerance is incomplete, the calculated equianalgesic dose of any new opioid is always lower than expected. One must be particularly cautious, however, when converting from morphine to methadone as the morphine/methadone equianalgesic ratio appears to be curvilinear; whereas the morphine-to-methadone conversion ratio is 3:1 at morphine doses of <100 mg/day, the ratio is 20:1 at morphine doses of >1,001 mg/day (Table 56-8). Methadone is principally metabolized by the CYP3A4 subtype enzyme of the cytochrome P450 system and to a lesser extent by the CYP1A2 and CYP2D6 subtypes. Consequently, there is the potential for numerous drug interactions with methadone, as shown in Table 56-9. Whereas inhibition of methadone metabolism will theoretically provoke toxicity, induction of methadone metabolism could potentially precipitate inadequate analgesia or even withdrawal symptoms. Frequent adjustments of the methadone dosage may therefore be required if medications are added to or eliminated from a patient’s drug regimen. A rare side effect associated with methadone is a pause-dependent dysrhythmia associated with bradycardia, QT prolongation, and Torsades de point.
TABLE 56-8. CONVERSION RATIOS FROM MORPHINE TO METHADONE
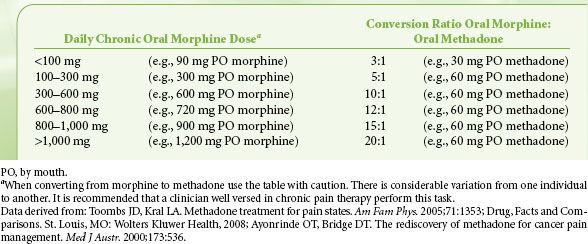
TABLE 56-9. METHADONE DRUG INTERACTIONS
Buprenorphine is a potent semisynthetic opioid that is derived from thebaine. The drug is classified as a mixed agonist–antagonist and partial mu receptor agonist with an analgesic potency 25 to 50 times greater than that of morphine. Buprenorphine is a lipophilic opioid with moderate intrinsic activity and a high affinity for the mu opioid receptor, with a half-life for dissociation from the receptor of 166 minutes compared with 7 minutes for fentanyl. The drug is metabolized by the gut and the liver and has a half-life of about 3 hours, but this bears little connection to the rate of disappearance of its clinical effects because of its avid binding to the mu receptor, as previously noted. The drug can be delivered by various routes of administration include intravenous, intramuscular, neuraxial, subcutaneous, sublingual, and transdermal. Buprenorphine is also an excellent alternative for the treatment of acute pain in the patient who cannot tolerate morphine secondary to allergy or other sensitivity. In humans, buprenorphine is reported to have a ceiling effect for respiratory depression but not for analgesia. Buprenorphine is reported to have anti-inflammatory effects and therefore may be efficacious when administered intra-articularly. Investigators have demonstrated that buprenorphine will significantly prolong the analgesic effects of a peripheral nerve block when 0.3 mg of the drug is combined with 40 mL of a local anesthetic mixture consisting of 1% mepivacaine, 0.2% tetracaine, and epinephrine 1:200,000.23
NONOPIOID ANALGESIC ADJUNCTS
The NSAIDs are among the most commonly used drugs in the world because of their anti-inflammatory, analgesic, and antipyretic effects (Table 56-10).  The therapeutic benefit of NSAIDs is believed to be mediated through the inhibition of cyclooxygenase (COX) enzymes (prostaglandin H2 synthetases), types 1 and 2, which convert arachidonic acid to prostaglandin H2 (PGH2). The COX enzyme consists of two active sites: (1) The cyclooxygenase site and (2) the peroxidase site. NSAIDs mediate their effects by binding to the cyclooxygenase site (Fig. 56-11)
The therapeutic benefit of NSAIDs is believed to be mediated through the inhibition of cyclooxygenase (COX) enzymes (prostaglandin H2 synthetases), types 1 and 2, which convert arachidonic acid to prostaglandin H2 (PGH2). The COX enzyme consists of two active sites: (1) The cyclooxygenase site and (2) the peroxidase site. NSAIDs mediate their effects by binding to the cyclooxygenase site (Fig. 56-11)
FIGURE 56-11. From Kam, P.C.A., COX-3: Uncertainties and controversies, Curr Anaesth Crit Care. 2009; (20) 50.
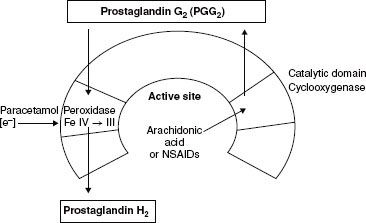
COX-1 is the constitutive enzyme that produces prostaglandins, which are important for general “house-keeping” functions such as gastric protection and hemostasis. COX-2, on the other hand, is the inducible form of the enzyme that produces prostaglandins that mediate pain, inflammation, fever, and carcinogenesis. Prostaglandin E2 is the key mediator of both peripheral and central pain sensitization. Peripherally, prostaglandins do not directly mediate pain; rather, they contribute to hyperalgesia by sensitizing nociceptors to other mediators of pain sensation such as histamine and bradykinin.24 Centrally, prostaglandins enhance pain transmission at the level of the dorsal horn by (1) increasing the release of substance P and glutamate from first-order pain neurons, (2) increasing the sensitivity of second-order pain neurons, and (3) inhibiting the release of neurotransmitters from the descending pain-modulating pathways.
NSAIDs have proved effective in the treatment of postoperative pain. In addition, they are opioid-sparing and can significantly decrease the incidence of opioid-related side effects such as postoperative nausea and vomiting and sedation.25 Unlike the opioids, NSAIDs exhibit a “ceiling effect” with respect to maximum analgesic effects. Parenteral NSAIDs such as ketorolac are commonly employed as part of a multimodal approach for acute perioperative pain management. The optimal dose of ketorolac for postoperative pain control is 15 to 30 mg intravenously every 6 to 8 hours not to exceed 5 days. The dose should be decreased in patients with renal failure.
Despite the benefits of NSAIDs in the perioperative period they are not without some significant side effects. Platelet dysfunction, gastrointestinal ulceration, and an increased risk of nephrotoxicity are several reasons why the nonselective NSAIDs may be avoided in the perioperative period. The risk of nephrotoxicity is increased in patients with hypovolemia, congestive heart failure, and chronic renal insufficiency.24 The COX-2–selective inhibitors were developed in an attempt to minimizing their side effects. The COX-2–specific inhibitor celecoxib (Celebrex) is available in the United States. Rofecoxib (Vioxx) and valdecoxib (Bextra) also released in the same period were recalled by the manufacturers because of concerns about adverse cardiovascular risks. Celecoxib is the only COX-2–specific inhibitor currently available in the United States for acute postoperative pain. The recommended oral loading dose is a 400 mg followed by 200 mg orally every 12 hours for several days. Parecoxib (Dynastat) is an injectable COX-2–specific inhibitor that is available only in Europe for the treatment of moderate-to-severe postoperative pain. The recommended dose of the drug in Europe is 40 mg intravenously or intramuscularly initially followed by 20 to 40 mg every 4 to 6 hours not to exceed 80 mg/day. Unlike the nonselective NSAIDs, however, COX-2–specific inhibitors offer the potential advantages of a reduced incidence of gastrointestinal ulceration and they do not inhibit platelet function. Because prostaglandins play a crucial role in renal function through their effect on blood flow, natriuresis and glomerular filtration, traditional NSAIDs, and the COX-2 inhibitors can cause fluid retention and hypertension.
 Short-term use of parecoxib and valdecoxib in patients following coronary artery bypass surgery is associated with an increased risk of thromboembolic events.26
Short-term use of parecoxib and valdecoxib in patients following coronary artery bypass surgery is associated with an increased risk of thromboembolic events.26
The authors, therefore, do not recommend prescribing a COX-2 inhibitor for patients with a known history of coronary artery disease or cerebrovascular disease. Both COX-1 and COX-2 play significant roles in bone fusion following fracture, and the use of the traditional NSAIDs has been found to inhibit the healing process, particularly following lumbar spinal fusion surgery. The effect of COX-2 inhibitors on bone fusion following orthopedic procedures continues to be controversial, and no recommendations can be made at this time. NSAIDs and COX-2–selective inhibitors should not be administered to patients with known hypersensitivity to the drugs or to patients with Samters triad (aka aspirin triad), which is a medical condition consisting of asthma, aspirin insensitivity, and nasal polyposis. Finally, avoid celecoxib and valdecoxib in patients with allergic-type reactions to sulfonamides.
TABLE 56-10. NONOPIOID ANALGESICS (ADULT DOSING GUIDELINES)

The para-aminophenol derivative acetaminophen (aka paracetamol) has both analgesic and antipyretic properties, similar to aspirin, but is devoid of any anti-inflammatory effects. The drug is primarily a centrally acting inhibitor of the cyclooxygenase enzyme with minimal peripheral effects. Acetaminophen neither enters the active site of the COX enzyme nor binds to the cyclooxygenase site, but instead it prevents COX activation by reducing heme at the peroxidase site of the enzyme (Fig. 56-11). In addition, there may be modulation of descending inhibitory serotoninergic pathways and the drug may act on the opioid, cannabinoid, and NMDA receptors.27 Acetaminophen is devoid of many of the side effects generally associated with the NSAIDs, such as gastrointestinal ulceration, impaired platelet function, adverse cardiorenal effects, and impairment of bone fusion following orthopedic procedures. Acetaminophen is opioid-sparing and can be used in conjunction with an NSAID as part of a multimodal analgesic program. In adults, 2 g of oral acetaminophen is equivalent to 200 mg of celecoxib.
Propacetamol is the intravenous prodrug formulation of paracetamol and is a popular adjuvant for perioperative pain control in Europe. The drug has the disadvantage that it must be reconstituted prior to administration. Two grams of propacetamol is equivalent to 1 g of paracetamol. Fortunately, a ready-to-use intravenous formulation of paracetamol (Perfalgan) has been released in Europe. Intravenous acetaminophen (Ofirmev) was released in the United States in November of 2010. The drug is available as a 1 g (1,000 mg/100 mL) infusion that does not require reconstitution and can be infused through a peripheral IV over 15 minutes. See Table 56-10 for dosing guidelines.
The NMDA receptor antagonists such as ketamine and dextromethorphan may be useful analgesic adjuncts. Excitatory neurotransmitter stimulation of the NMDA receptor is believed to be involved in the development and maintenance of several phenomena including (1) persistent postoperative pain, (2) hypersensitivity, wind-up, and allodynia, (3) opioid-induced tolerance, and (4) OIH. Low-dose ketamine (0.25- to 0.5-mg intravenous bolus followed by an infusion of 2 to 4 μg/kg/min) can provide significant analgesia and is opioid-sparing. The mechanism of action of ketamine is NMDA receptor blockade, but in addition the drug interacts with opioidergic, cholinergic, and monoaminergic receptors and blocks sodium channels.28
NMDA receptor antagonists may act synergistically when combined with an opioid. The ideal intravenous PCA morphine–ketamine combination ratio is 1:1 with an 8-minute lockout.29 A double-blind study, however, demonstrates that the combination of ketamine (1 mg/mL) with morphine (1 mg/mL) administered as an intravenous PCA to patients following major abdominal surgery does not significantly improve pain relief.30 In patients with morphine-resistant pain, however, the combination of 250 μg/kg of ketamine plus 15 μg/kg of morphine has been reported to provide significant analgesia.28 Results are promising, but more studies will certainly be required to clearly define the role of ketamine for postoperative analgesia.
Dextromethorphan, the d-isomer of the codeine analog levorphanol, is a noncompetitive NMDA receptor antagonist that has been used for many years as an antitussive. Dextromethorphan does not have a direct analgesic effect; rather, analgesia is likely mediated by its NMDA receptor antagonism. The drug can be administered orally, intravenously, and intramuscularly. There is a sustained-release suspension available that contains dextromethorphan, 30 mg/5 mL, and is marketed as Delsym (Adams Respiratory Therapeutics). Following oral administration the drug is metabolized to dextrorphan, which is the metabolite that accounts for most of the side effects, the most common of which is nausea and vomiting. Because the intravenous administration of large doses can lead to hypotension and tachycardia, the intramuscular route may be the preferred route of delivery. Dextromethorphan has been shown to both inhibit secondary hyperalgesia following peripheral burn injury and cause a reduction in temporal summation of pain. The preoperative administration of 150 mg of oral dextromethorphan can reduce the PCA morphine requirements of patients undergoing abdominal hysterectomy, and the preincisional administration of 120 mg of intramuscular dextromethorphan provides preemptive analgesia in patients undergoing elective upper abdominal surgery. Finally, a randomized double-blind placebo-controlled study has demonstrated that dextromethorphan dosed 200 mg orally every 8 hours (e.g., 2 hours prior to surgery, then 8 hours and 16 hours thereafter) can provide a modest reduction in morphine consumption following knee surgery.31
The α2-adrenergic agonists clonidine (half-life, 9 to 12 hours) and dexmedetomidine (half-life, 2 hours) may be administered perioperatively to provide analgesia, sedation, and anxiolysis. The presynaptic activation of α2-receptors that results in the decreased release of norepinephrine is believed to mediate analgesia. Whereas clonidine is a selective partial agonist for the α2-adrenoreceptor, dexmedetomidine is super selective for the receptor. Their respective α2/α1 binding ratios are 220:1 for clonidine versus 1,620:1 for dexmedetomidine. Analgesia is mediated supraspinally (locus coeruleus), spinally (substantia gelatinosa), and peripherally. Dexmedetomidine is reported to have greater affinity for the 2A subtype of the receptor, which may account for the drug’s superior analgesic properties vis-à-vis clonidine. Clonidine can be administered orally, transdermally, intravenously, perineurally, and neuraxially for perioperative pain management. Premedication with 5 μg/kg of oral clonidine in patients undergoing knee surgery can decrease the use of PCA morphine and decrease the incidence of postoperative nausea and vomiting. In addition, the combination of oral clonidine, 3 to 5 μg/kg with 0.2 mg/24 hours of transdermal clonidine can decrease postoperative PCA morphine requirement by 50% following prostatectomy surgery. In a double-blind placebo-controlled study, investigators demonstrated that addition of 25 μg of intrathecal clonidine to a bupivacaine (15 mg) morphine (250 μg) spinal anesthetic cocktail, for total knee arthroplasty (TKA), could reduce postoperative morphine use and improve VAS pain scores at 24 hours.32 Clonidine in doses of 0.5 to 1 μg/kg may enhance the efficacy and increase the duration of local anesthetics in peripheral nerve blockade. In addition, the intra-articular use of clonidine can be beneficial. Side effects include sedation, hypotension, and bradycardia if the dose exceeds 150 μg as part of a peripheral nerve block.
Dexmedetomidine is the D-enantiomer of medetomidine. It is a highly selective α2-agonist that does not interact with the GABA-mimetic system and therefore does not depress the respiratory drive.33 Other advantages include analgesia, titratable sedation (e.g., “cooperative sedation”), and anxiolysis. In addition, the centrally mediated reduction in sympathetic tone is reported to have a cardioprotective effect, particularly in high-risk patients undergoing vascular surgery. There is a low incidence of bradycardia and hypotension, which can be treated with atropine, ephedrine, or volume infusion.33 Dexmedetomidine is a useful adjunct to both opioid and nonopioid analgesics as part of a multimodal analgesic protocol. Combining the drug with ketamine and opioids can obviate the respiratory depressant effects of the latter and the psychomimetic effects of the former.34 The recommended dose of dexmedetomidine is a loading dose of 1 μg/kg intravenously over 10 minutes followed by an infusion of 0.2 to 0.7 μg/kg/hr,33 A dexmedetomidine infusion (0.2 to 0.7 μg/kg/hr) combined with peripheral nerve blockade can provide superb analgesia, anxiolysis, and sedation during prolonged procedures.
The gabapentinoids (α2–δ subunit calcium channel ligands), gabapentin (Neurontin) and pregabalin (Lyrica), are indicated for the treatment of partial onset seizures, neuropathic pain (e.g., postherpetic neuralgia), and other chronic pain states (e.g., fibromyalgia) but have been commonly prescribed for off-label use for acute perioperative pain relief. Numerous meta-analysis indicates that the perioperative administration of gabapentinoids improves postoperative pain (both at rest and with movement) and decreases postoperative opioid requirement. In addition, there is a decreased incidence of postoperative vomiting, pruritus and urinary retention probably secondary to the opioid-sparing effects of the drugs.35
Compared to traditional analgesics, which decrease afferent input from the site of tissue injury, the gabapentinoids decrease the hyperexcitability of dorsal horn neurons caused by tissue damage.36 Although structurally similar to GABA (gamma-aminobutyric acid) these drugs are not GABAergic and do not bind GABAA GABAb GABAc radioligand sites or allosteric GABA receptor sites. The analgesic mechanism of action of the gabapentinoids may involve 3 distinct processes: (1) Decreased release of neurotransmitter via presynaptic binding of the calcium receptor (Cav α2 δ), (2) redistribution of the calcium channel away from its functional membrane bound site to its nonfunctional cytosolic site, and (3) inhibition of activation of the transcription factor nuclear factor κB (NF- κB), which reduces gene transcription of COX-2 and interleukin-6 (IL-6).37
Gabapentin prevents the development of central excitability and is antihyperalgesic. Meta-analysis of the analgesic effects of gabapentin suggests that it should be part of any multimodal analgesic regimen for perioperative pain management.38 In a double-blinded, randomized, placebo-controlled study, gabapentin was administered as a 1,200-mg oral dose 1 to 2 hours prior to surgery in patients scheduled to undergo elective arthroscopic anterior cruciate ligament (ACL) repair.39 Advantages of this regimen included a 50% reduction in postoperative morphine consumption and improved early knee flexion following surgery. In another double-blind placebo-controlled study,40 patients undergoing abdominal hysterectomy reported superior postoperative analgesia following the administration of gabapentin 1 to 2 hours preoperatively. The recommended adult dose of gabapentin as part of a multimodal pain regimen for postoperative pain management is 900 mg orally 1 to 2 hours prior to surgery.40 The gastrointestinal absorption of gabapentin occurs through a saturable transport system resulting in bioavailability that decreases with increasing doses. Therefore, increased doses of the drug result in incrementally smaller increases in plasma drug concentration (e.g., nonlinear pharmacokinetics).41 Common side effects include dizziness, drowsiness, and ataxia.
Pregabalin is a newer gabapentinoid with greater potency than gabapentin that may be useful for the treatment of acute perioperative pain. A recent meta-analysis suggests that pregabalin has significant opioid-sparing effects in the first 24 hours following surgery and postoperative vomiting is reduced.36 There is also strong evidence suggesting that the perioperative use of pregabalin can reduce the incidence of chronic neuropathic pain following TKA and can reduce the time necessary to achieve effective joint range of motion.42 Compared to gabapentin, pregabalin has a linear pharmacokinetic profile (dose-independent absorbtion), increased potency, and fewer side effects. Further investigation will be required to better define useful drug combinations and dosing regimens for gabapentin and pregabalin.
The local anesthetic lidocaine has been shown to be analgesic, antihyperalgesic, and anti-inflammatory following intravenous administration.43 The perioperative infusion of lidocaine has been shown to not only improve postoperative analgesia in patients recovering from laparoscopic colectomy, but it also can decrease postoperative opioid requirements, attenuate postoperative ileus, and accelerate time to discharge from the hospital.43 Further studies are certainly warranted to establish both the safety and efficacy of this novel approach.
The glucocorticoids are well known for their analgesic, anti-inflammatory, and antiemetic effects. Inhibition of cytosolic phospholipase A2 upstream from the lipoxygenase and COX enzymes in the prostaglandin cascade most certainly accounts for both their anti-inflammatory and analgesic effects by inhibiting leukotriene and prostaglandin production. The mechanism of the antiemetic effect of the corticosteroids is less clearly understood but appears to be centrally mediated.44 Although corticosteroids are well recognized as effective analgesics following oral surgery, the analgesic benefits following general surgery, orthopedic surgery, and back surgery have had mixed reviews. Results from a recent study suggest that the combination of dexamethasone (8 mg intravenously) and gabapentin (800 mg orally) administered 1 hour prior to varicocele surgery improves postoperative analgesia and decreases the incidence of postoperative nausea and vomiting.44 Side effects associated with the use of corticosteroids include gastrointestinal upset, impairment of the immune system, and delayed wound healing; however, because of the small doses used perioperatively, corticosteroids are considered to be relatively safe. Future studies will better define the role of corticosteroids in perioperative pain management.
METHODS OF ANALGESIA
Patient-controlled Analgesia
PCA is any technique of pain management that allows the patients to administer their own analgesia on demand. We will highlight some important aspects of PCA as a complete review of PCA is beyond the scope of this chapter; we refer the reader to the comprehensive review by Macintyre on this topic.45 In the United States, the most commonly used drugs are morphine, hydromorphone, and fentanyl. Hydromorphone is recommended as an alternative in renal failure; however, fentanyl might be a better choice as it has no active metabolites. Meperidine is not recommended for use in an intravenous PCA secondary to accumulation of its potentially toxic metabolite normeperidine.
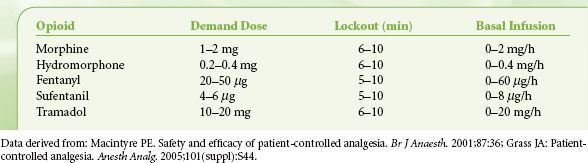
 The five variables associated with all modes of PCA include (1) bolus dose, (2) incremental (demand) dose, (3) lockout interval, (4) background infusion rate, and (5) 1- and 4-hour limits. A typical PCA regimen in an otherwise healthy adult would be an incremental dose of 1 to 2 mg of morphine with an 8- to 10-minute lockout (Table 56-11). The authors do not recommend a background infusion of opioid in the opioid-naive patient. A background infusion should be reserved for the patient with chronic malignant or nonmalignant pain who is opioid-tolerant or in patients with persistent pain who have failed a trial of incremental PCA dosing. In the elderly, the dose of the PCA should be decreased. The relative risk factors for use of an opioid PCA are listed in Table 56-12. If more than two risk factors exist, you may want to avoid using a PCA in the standard dosing regimen and administer opioids only as needed.
The five variables associated with all modes of PCA include (1) bolus dose, (2) incremental (demand) dose, (3) lockout interval, (4) background infusion rate, and (5) 1- and 4-hour limits. A typical PCA regimen in an otherwise healthy adult would be an incremental dose of 1 to 2 mg of morphine with an 8- to 10-minute lockout (Table 56-11). The authors do not recommend a background infusion of opioid in the opioid-naive patient. A background infusion should be reserved for the patient with chronic malignant or nonmalignant pain who is opioid-tolerant or in patients with persistent pain who have failed a trial of incremental PCA dosing. In the elderly, the dose of the PCA should be decreased. The relative risk factors for use of an opioid PCA are listed in Table 56-12. If more than two risk factors exist, you may want to avoid using a PCA in the standard dosing regimen and administer opioids only as needed.
TABLE 56-11. USUAL INTRAVENOUS OPIOID PATIENT–CONTROLLED ANALGESIA REGIMENS IN THE OPIOID-NAIVE ADULT PATIENT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






