CHAPTER 29. Diving Emergencies
Reneé Semonin Holleran
COMPETENCIES




1. Perform a comprehensive assessment of the patient, including subjective and objective data on the diving emergency.
2. Anticipate and plan for the effects of transport on the patient with decompression illness.
3. Contact the Divers Alert Network website (http://www.diversalertnetwork.org) for advice on the care of the patient with a diving emergency.
Scuba (from “self-contained underwater breathing apparatus”) diving is an increasingly popular pastime. The United States is now estimated to have more than nine million certified divers, with many more worldwide. 11 Diving activities are no longer restricted to coastal resorts but can be found in almost any body of water large enough to hold a diver and equipment. Diving is also a part of many occupations, including industry, military, scientific research, and search and rescue. Occasionally, an accident may even occur at a city aquarium, especially if envenomization is involved. 4,7
Diving can lead to illnesses and injuries, some of them unique to the environment. Injuries and fatalities from diving may be reported at the Divers Alert Network (http://www.diversalertnetwork.org). Generally, about 1000 injuries are reported each year; fatalities vary from year to year. 7
Manifestations of diving injuries may not be noticed by the diver for 24 to 48 hours after a dive and may, in fact, be seriously potentiated by air travel. Thus, patients with diving-related problems may be seen many hours later and many thousands of miles from the original dive site.
Transport personnel encounter many types of scuba-related diving injuries, such as marine envenomation, near drowning, decompression illness, arterial gas embolism (AGE), middle-ear squeeze, and other forms of barotrauma. Of these diving injuries, decompression illness and AGE are medical emergencies that necessitate immediate recompression treatment. Air medical transport of the patient to a hyperbaric chamber is often necessary to avoid the significant morbidity and mortality that result from delays in treatment of these disorders. Transport personnel must therefore be able to diagnose and manage these diving emergencies in a timely manner.
This chapter provides a brief discussion of diving principles and the pathophysiology, clinical manifestations, and management of diving emergencies likely to be encountered by transport crews.
DIVING PRINCIPLES
A brief discussion of a few physical properties inherent to scuba diving is necessary for a thorough understanding of the pathophysiology underlying decompression illness and air embolism.
At sea level, a 1-square-inch column of air extending upward from the earth’s surface to the edge of the atmosphere weighs 14.7 lb. Thus, the pressure exerted by this column of air at sea level is 14.7 lb/in 2 (psi) or 760 mm Hg, which is defined as 1 atmosphere of pressure (atm). As altitude increases, the column of air becomes shorter and the air pressure decreases. For example, at an altitude of 18,000 ft, the atmospheric pressure is half that at sea level: 380 mm Hg, or 0.5 atm. On the other hand, water is much denser than air, and a similar 1-square-inch column in seawater only has to be 33 ft (10 m) to exert the same amount of pressure as a 1-square-inch column of air. Because the density of water is uniform throughout, the proportional relationship of pressure and depth remains constant: pressure increases 1 atm for every 33-ft (10-m) column of seawater (Figure 29-1). For the scuba diver, the combined weights of the air and water columns must be taken into consideration. At a given depth underwater, the total pressure is the sum of the barometric pressure exerted by the column of air above plus the hydrostatic pressure exerted by the column of water. This total is the concept of absolute pressure or atmospheres absolute (ATA). Therefore, a scuba diver at a depth of 33 ft experiences an ambient pressure of 2 atm absolute pressure, or 2 ATA. Similarly, a scuba diver at 66 ft experiences an ambient pressure of 3 ATA.
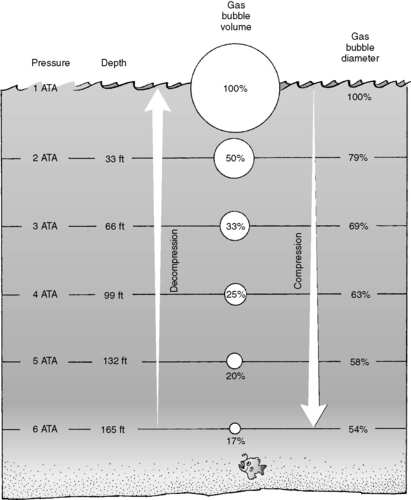 |
| FIGURE 29-1 Boyle’s law. (From Auerbach PS: Wilderness medicine: management of wilderness and environmental emergencies, ed 4, St Louis, 2001, Mosby.) |
As the diver descends from the water’s surface, the effects of increasing ambient pressure on the scuba diver involve an understanding of the behavior of gases under conditions of varying pressure and volume. The following brief discussion concerns the primary gas laws of diving. 1,10,13,17,18
BOYLE’S LAW
The first gas law is Boyle’s law, which states that at a constant temperature and mass, the volume of a gas is inversely proportional to the total pressure. Simply stated, volume decreases as pressure increases; conversely, volume increases as pressure decreases. Figure 29-1 depicts the increase of gas volume as the pressure and depth decrease.
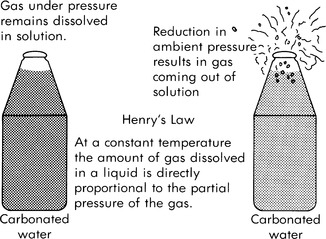 |
| FIGURE 29-2 Henry’s law. |
HENRY’S LAW
The second gas principle is Henry’s law, which states that solubility is proportional to the partial pressure of a gas. As the pressure increases or decreases, the gas goes into or comes out of solution accordingly. This effect is the “soda bottle” phenomenon. When you release the pressure from the bottle by removing the cap, the dissolved gas comes out of solution (Figure 29-2).
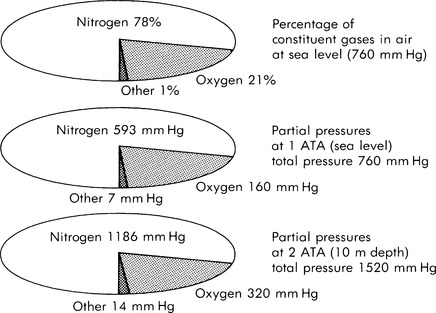 |
| FIGURE 29-3 Dalton’s law. |
DALTON’S LAW
The last gas principle is Dalton’s law of partial pressures, which states that the total pressure of a mixture of gases equals the sum of partial pressures exerted by the constituent gases. The partial pressure is the pressure exerted by a single gas in a mixture as if it were the only gas in the mixture. Air comprises approximately 78% nitrogen, 21% oxygen, and 1% other gases. As illustrated in Figure 29-3, with an increase in the total pressure of the mixture, the pressure of each constituent gas is increased proportionately. At a depth of 99 ft (30 m), a scuba diver is under an ambient pressure of 4 ATA and is breathing compressed air with partial pressures of nitrogen and oxygen four times their value at the surface.
PATHOPHYSIOLOGIC FACTORS
Nitrogen is a relatively inert gas that is driven into solution according to Henry’s law as the diver descends. The saturation of tissues with nitrogen depends on intrinsic properties, including tissue perfusion and solubility coefficients of the various tissues. 11 The quantity of dissolved nitrogen in the tissues increases with the duration and depth of the dive. 8 If ascent of the scuba diver is too fast or the tissues are oversaturated with gas, the nitrogen is separated from solution rather than safely transported to the lungs for elimination. Tissue desaturation of nitrogen results in the formation of inert gas bubbles in venous blood and tissues on reduction in ambient pressure. 11
DECOMPRESSION ILLNESS
The lesion that results from decompression illness has been a subject of debate for many years, although tissue ischemia is generally accepted to be the final common pathway. 8,11,19 Hallenbeck, Bove, and Elliott9 demonstrated venous congestion with gas bubbles in the epidural venous plexus system of the spinal cord, most frequently in the lumbosacral region. The location of the intravascular lesion was consistent with the corresponding neurologic symptoms. 9 The formation of bubbles in tissues and venous blood has multiple mechanical and physiologic consequences. Mechanical effects of bubble formation include intravascular or intralymphatic obstruction, cellular distention and rupture, and stretching of ligaments and tendons. These effects result in ischemia or infarction, edema formation, cell death, and pain. The physiologic consequences include activation of the intrinsic clotting pathway, kinins, and the complement system, which all result in platelet aggregation, increased vascular permeability, and microvascular sludging. The end results of all of these events are decreased tissue perfusion and ischemia. 11
ARTERIAL GAS EMBOLIZATION
By far the most serious manifestation of pressure-related injuries or barotrauma is arterial gas embolization (AGE). The most classic presentation is a sudden onset of unconsciousness within minutes of reaching the surface after a dive. AGE is a leading cause of death among scuba divers. 2,5,11.12.13.14.15.16.17. and 18.
Divers need to exhale continuously and ascend slowly, or several consequences may occur. These consequences include the following:
▪ Air pushes through the lung tissues and enters the skin in the neck.
▪ Air pushes through the lung tissues and into the spaces between the lungs and causes a pneumothorax.
▪ Air is forced from the lungs into blood vessels and carried to vital organs.
In accordance with Boyle’s law, gases in the lungs of a scuba diver expand as ambient pressure decreases during ascent. The greatest changes in pressure and volume occur at shallower depths. Pulmonary overpressurization syndrome and alveolar rupture can occur during an ascent from a depth as shallow as 4 ft if compressed air is held in the lungs. 1 Breath holding during an ascent, as with a panicked diver, or air trapping in a diseased lung results in lung overexpansion and rupture of alveoli. Air bubbles from the ruptured alveoli are free to enter the pulmonary venous return to the left side of the heart for subsequent dissemination through the systemic circulation. Air bubbles can track in the lung parenchyma and tissue planes. The result may be interstitial and mediastinal emphysema or pneumothorax. 1
Gas bubbles may enter the coronary arteries and produce myocardial ischemia or infarction. However, bubbles most often enter the carotid circulation, producing multiple areas of circulatory occlusion in the brain with resulting ischemia and infarction.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





