Introduction
Symptomatic orthopedic pain therapy concentrates on nociception in bones, muscles, tendons, and joints after the pain stimulus has already acted on the body. This means that the nociceptive process has already started. Nociceptors have been irritated, and the pain signals have been transmitted to the brain via the afferent fibers and the spinal cord. Pain has already been perceived in the brain, and the motor and autonomic reactions in the periphery have been initiated. Symptomatic pain therapy acts on the different areas responsible for the transmission and perception of pain, and the reaction to it, with the emphasis varying according to the individual type of treatment (Fig. 4.1.)
In symptomatic pain therapy, unlike causal pain therapy, the physician and patient expect an immediate response. Thus, fast-working analgesics, local injections, physical agents, and directly acting forms of electrotherapy form the main focus of treatment (Table 4.1).
| Therapeutic local injection treatment (TLIT) |
| Medication |
| Thermotherapy |
| Physical therapy |
| Electrotherapy |
| Acupuncture |
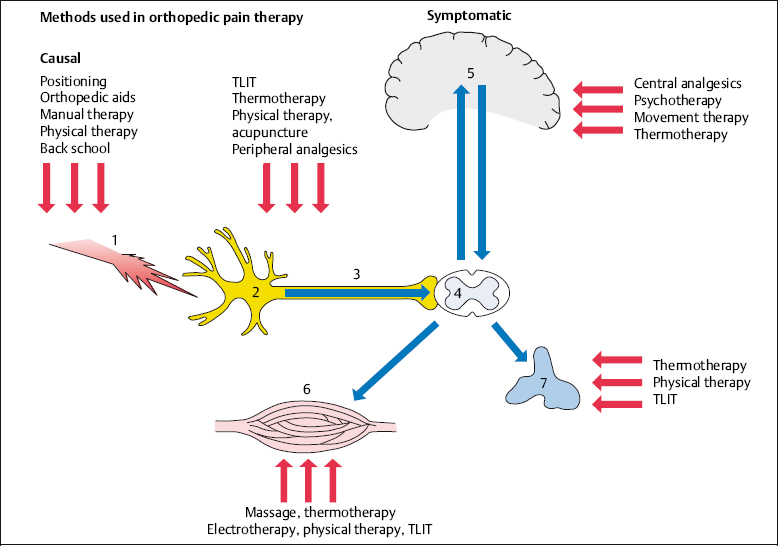
Fig. 4.1 Methods used in orthopedic pain therapy.
Thermotherapy
NOTE
All forms of warmth ease spinal pain.
In many cases patients apply soothing warmth before consulting a doctor. Warmth acts as a local analgesic, removing inflammatory mediators, relaxing muscles, and calming the autonomic nervous system. The cortex and the psyche perceive warmth as being pleasant (Fig. 4.2).
 Forms of Application
Forms of Application
Warmth can be applied in different ways. Heat can be transmitted either by placing the heat carrier in direct contact with the patient or indirectly by using radiant heat. The warmth from fango (volcanic mud used in spa treatments) and mud packs penetrates well into the skin. The simple application of dry heat, e. g., various forms of infrared treatment, has also proven effective in practice. Heat pads, hot water bottles, and hot baths are immediate measures which are recommended for home treatment. The local thermal effects are, in decreasing order (Tilscher 1989):
- steam shower 52°
- hot shower 40–42°
- hot packs 40–42°
- mud packs 43–45°
- hay packs 43–45°
- hot air treatment (hot air box) 35°
The steam shower is the most intensive form of local heat application and is especially pleasant for patients suffering from chronic recurring cervical syndromes.
Contraindications for the local and generalized application of heat are thromboses and thrombophlebitis, cardiovascular disorders, dermatoses, acute inflammation, and florid infectious processes.
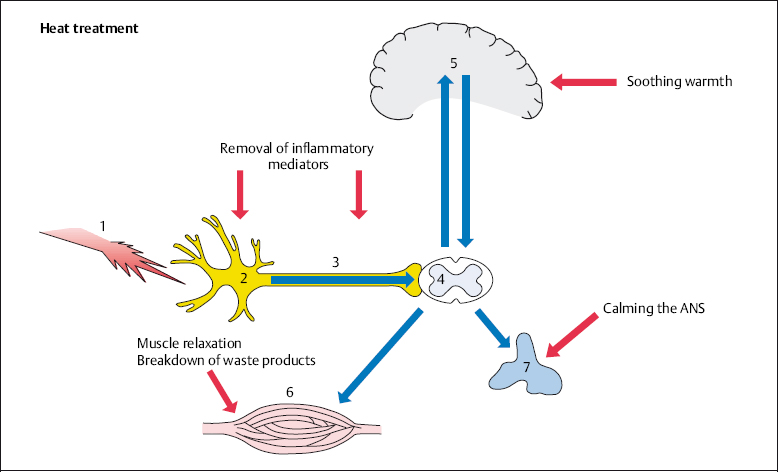
Fig. 4.2 The application of heat in the treatment of back pain.
Massage
NOTE
Massage is a special form of manual therapy used to treat painful spinal disorders.
The hands are used to massage skin, subcutaneous fatty tissue, muscles, and ligaments. A variety of techniques are applied during a massage:
- stroking and frictioning
- kneading and wringing
- cross-frictions.
Special forms of massage include reflex zone massage and connective tissue massage. The connective tissue massage technique involves a specific way of stroking with a fingertip (usually the third or fourth finger) with the hand, arm, and shoulder being held in a relaxed position. The direction of the strokes is segmentally orientated.
It is important that the patient is placed in a comfortable and relaxed position during all types of massage. This particularly applies to the affected body part. Massage acts positively on local nociception by removing inflammatory mediators. In addition, the vicious cycle of pain–muscle cramping–pain is disrupted at the muscular level (Fig. 4.3).
Contraindications to massage arise, as a rule, from acute pain, inflammation, skin changes, and nerve root compression syndromes.
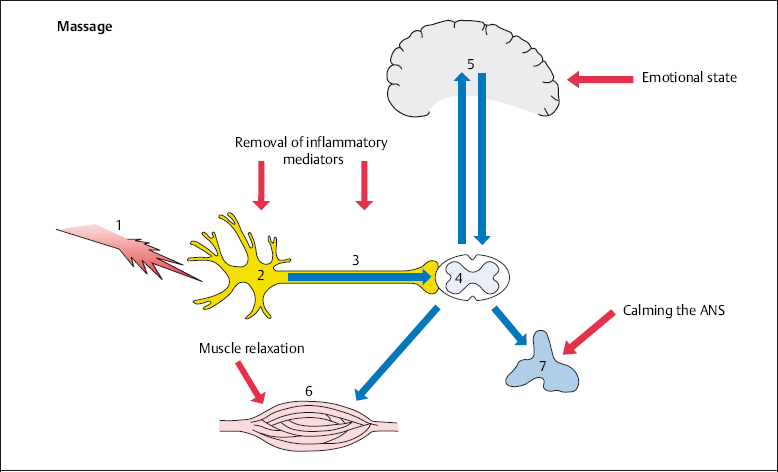
Fig. 4.3 Massage in spinal pain therapy.
Electrotherapy
In electrotherapy, electrical energy is used for the purpose of healing. Different types of currents vary in their physical and biological actions:
- High-frequency currents in the form of short waves, decimeter waves, and microwave therapy act by warming the depths of the tissue being treated. The oscillations are too fast to excite cells directly. The high-frequency currents that are applied therapeutically start at 20 000Hz, increase to the diathermic ranges of ~3 × 106 Hz, and go up to the short-wave therapeutic range at ~5 × 107Hz.
- Low-frequency currents (15–250 Hz) are used in galvanization, where direct current is applied. In this frequency range an increase in polarization or depolarization is found in the cells, with all transitional stages. A pain-relieving effect has been attributed to the constantly flowing direct current (e. g., in the form of a hydroelectric bath). Bernard’s diadynamic currents are low-frequency currents with alternating frequency and amplitude, which also act to relieve pain. The electric currents are usually applied bipolarly, i. e., using two electrodes.
- Mid-frequency currents are sinusoidal alternating currents with frequencies between 1 and 1000 Hz. The principle of mid-frequency or interferential current treatment involves the production of biologically effective frequency ranges within the organism itself. In interferential treatment, two biologically nonirritant mid-frequency currents (e.g., 4000 Hz) are applied to the body using two electrodes. The frequency of the currents differs by up to 100 Hz. Their superposition results in the development of an amplitude- and frequency-modulated current with lower effective frequency, i. e., a frequency that is biologically effective within the body. The advantages of interferential treatment are twofold: the mid-frequency stimulating current effectively overcomes the pain-sensitive skin and outer tissue layers, while the low-frequency currents (between 0 and 100Hz) are first generated in the deeper tissue layers and function there as a form of pain therapy. In this way, electrical currents that cannot be applied directly from external sources at this frequency and intensity are developed at the desired location within the body. The direct action of the low-frequency current in the deeper tissue affects the autonomic nervous system and improves blood flow.
High-frequency, low-frequency, and interferential therapy act similarly on nociceptors, afferent fibers, muscles, and the autonomic nervous system (Figs. 4.4, 4.5).
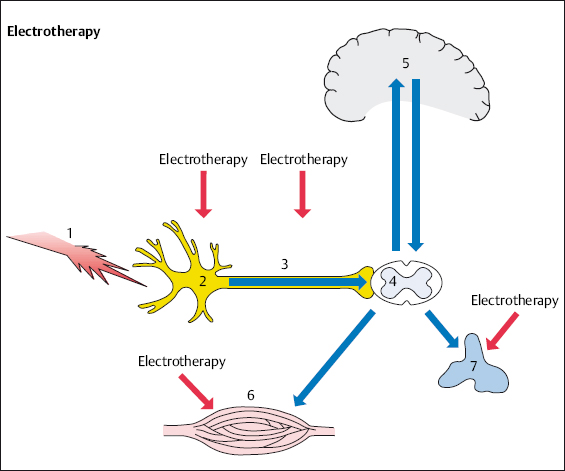
Fig. 4.4 Electrotherapy in spinal pain therapy.
Acupuncture
The word acupuncture comes from the Latin acus, “needle,” and pungere, “to prick.” Both traditional and classical Chinese medicine use a technique known as zhēn jiŭ (= insertion and burning, referring to acupuncture together with moxibustion). Fine needles are inserted into specific points on the body. The Chinese term for these acupuncture points is shu xue, where shu means transporting or conducting, and xue means cavity or hole.
NOTE
Acupuncture is the practice of inserting needles into specific areas of the skin. Among other effects, it activates the body’s own pain inhibition.
According to Heine (1987), ~80% of the traditional acupuncture points have an anatomical counterpart. Morphologically they can be described as perforations of the superficial body fascia tissue by a bundle of blood vessels and nerves.
The analgesic effect of acupuncture is probably due to the release of endorphins, which ease pain. According to Pomeranz (1981), the peripheral pain stimulus activates the analgesic action. This gives rise to a mechanism that acts in three stages. The pain caused by the needle prick acts as a noxious stimulus (1 in Fig. 4.6), stimulating the nociceptor (2). The pain signals are then further transmitted to the posterior horn of the spinal cord (4) via the afferent fibers (3). It is here that the pain signals are transferred to a second neuron, which in turn transmits the pain signals up to the thalamus and finally to the cortex (5), the place where pain is localized. The body’s own opioid peptides (endorphins) inhibit the transmission of nociceptive information, acting on the synapses of the nociceptive system in the spinal cord (4) and the brain (5). Endogenous opioids are released as inhibitory transmitters from the neurons. These neurons can be thought of as part of the antinociceptive system that is activated by acupuncture (Stux et al. 2003).
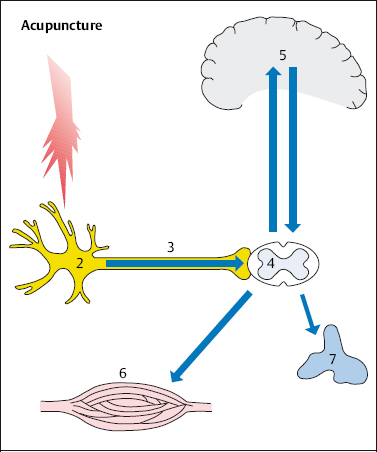
Fig. 4.6 Acupuncture and nociception. The pinprick from acupuncture acts as a noxious stimulus (1), stimulating the nociceptor (2). The stimulus is transmitted to the spinal cord (4) via the afferent fibers (3) and from there via the spinothalamic tract to the brainstem (5). Pain is not perceived. Pain-inhibiting mechanisms are sent to the periphery via the descending pathways and act as an analgesic.
The classic body acupuncture can be used to treat all forms of chronic pain, acting an adjuvant form of treatment within orthopedic pain therapy. The treatment should preferably be performed in a stress-relieving position, i. e., with the spine relaxed (Fig. 4.7).
We have assessed pain patients during a comparative study on the effectiveness of acupuncture within the scope of pain therapy. In this study acupuncture points were not individually chosen, but were rather based on standardized points for needle insertion (Grifka and Schleuß 1995). The study established that acupuncture using the traditional acupuncture points is significantly more effective than placebo acupuncture, where points were randomly chosen. The reported values for the total amount of pain and the highest level of pain were significantly lower for patients treated with traditional acupuncture over 14 treatment sessions than for patients treated with placebo acupuncture (Grifka and Schleuß 1995). However, the attitude of individual patients to the effectiveness of acupuncture plays an important role (Grabow 1992). A meta-analysis demonstrated that 75 out of 88 studies showed a positive result when acupuncture was used in pain therapy (Molsberger and Böwing 1997).
At least 10 acupuncture treatment sessions of at least 15 minutes each are required for effective treatment.
Therapeutic Local Injection Treatment
NOTE
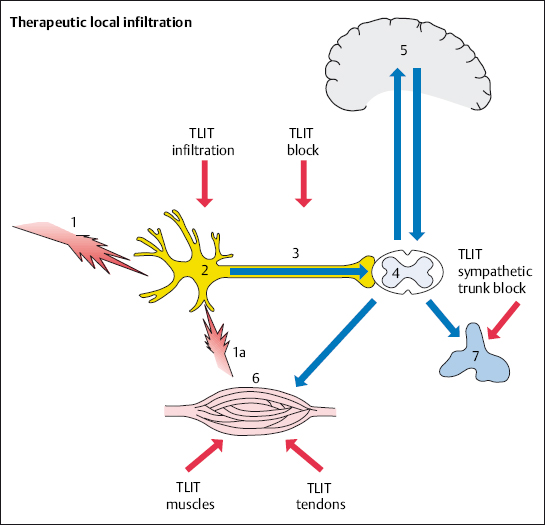
It is possible to treat the primary disorder by injecting fluids with anesthetic, anti-inflammatory, and anti-edemic properties directly into the nociceptive source in the spine (Fig. 4.8). This avoids loading the entire body with more medication than necessary.
The patient’s medical history and the results of manual medicine examinations provide important clues to the choice of site for the local therapeutic injection (see Chapter 2, “Clinical Examination”). Further guidance can be obtained from diagnostic local anesthesia or local pain provocation using saline solutions or contrast agents (see Chapter 2, “Trial Measures for the Diagnosis of Pain”).
Local anesthetics, steroids, or a combination of the two are used for the therapeutic local injection, depending on the aim of the treatment. Pure saline solution is also sometimes used: the hypertonic solution has an osmotic effect on the edemic tissue and dilutes the accumulated inflammatory mediators.
Therapeutic local anesthesia (TLA) is a fundamental part of the therapeutic local injection treatment (TLIT). A few milliliters of dilute (0.5–1.5%) local anesthetic solution are all that is needed to switch off sensitized nociceptors and nerve fibers that have developed into nociceptors. This results in the following effects:
- pain reduction
- decreased nerve excitability
- increased local blood flow.
Nociceptors and afferent fibers are reversibly switched off after local anesthetics have infiltrated the tissue. This abolishes the excitability of pain transmission, sensitive end organs, and the ability of sensitive sections of nerve fibers to transmit signals, and does so in a reversible manner at a local level. The effectiveness of local anesthesia decreases as nerve fiber diameter increases. For this reason, sensitive nerves are initially blocked and motor nerve fibers are blocked when high doses are injected. Therapeutic local anesthesia is directed toward the sensitive nerve fibers. Local anesthetics reduce the permeability of the membrane to cations, especially sodium ions. This results in diminished membrane permeability with reduced levels of excitability.
NOTE
The use of high concentrations with complete anesthesia and paralysis is not necessary during the local infiltration treatment. The aim is to decrease excitability and increase irritation thresholds.
Neurophysiologically based TLA measures break the link between muscle tension and the excitation of nociceptors (Zimmermann 1993). A nociceptor or nerve block results in a reduction in pain and nerve excitability and an increase in local blood flow for a period of 3–8 hours, depending on how long the applied local anesthetic works effectively. Experience shows that the pain-relieving effect is maintained longer than would be expected from the local anesthetic’s duration of action. This is especially the case with repeated administration. The state of reduced excitability continues, so that it is possible to obtain a permanent effect with a series of 8–12 infiltrations on consecutive days.
Infiltrating a local anesthetic multiple times into the area of nociception and the outgoing afferent fibers results in a desensitization of overactive neural elements. The frequency and intensity of the transmitted excitatory impulses that are required for pain perception and motor or autonomic reactions decline.
If chronification has already occured, the use of repeated TLA disrupts the vicious circle of adaptive posture–nerve irritation–increased muscle tension and pain at the neural level. The desensitization of nociceptors and afferent fibers, with an increase in irritation thresholds, leads to the same mechanical stimuli causing less pain. In this phase causal pain therapy has to be implemented by relieving positioning, exercises, etc. In chronic spinal pain syndromes, the repeated use of TLA in the area of nociception and afferent fibers results in a reduction in pain perception and pain processing (Zieglgänsberger 1986).
Rydevik’s (1990) and Olmarker’s (1993) groups studied the reactive inflammatory changes in nerves and nerve roots (e. g., due to prolapsed intervertebral disk tissue). They demonstrated that a defined chronic compression evokes an inflammatory and edematous change in the nerve root. This change can be largely prevented by the use of lidocaine injections (Yabuki et al. 1996).
Most local anesthetics also act as vasodilators, so blood flow increases markedly in the infiltrated area. However, this also means that injected medication is more rapidly removed by the circulatory system. In most cases, however, there is no indication for the addition of vasoconstrictors to the local injection treatment used for spinal symptoms. Notable specific side effects of the administration of local anesthetics are cardiovascular complications when the blood levels are too high, and allergic reactions. These complications are rare, however.
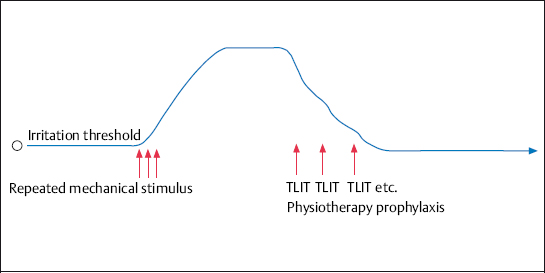
Fig. 4.9 The reduction in the excitability of nociceptors and afferent fibers by repeated use (8–12 times) of therapeutic local anesthesia. The irritation thresholds of nociceptors and afferent fibers, which have been raised by the repeated pain stimuli, revert to their normal level. The additional use of electrotherapy with positioning, physical therapy, and heat treatment further strengthens this effect.
A maximum of 10 mL of 0.5–1% local anesthetic is used at each injection treatment session in order to avoid elevated blood levels. Intravascular application is avoided by constant aspiration.
 Reliable Medications for Therapeutic Local Injection Treatment
Reliable Medications for Therapeutic Local Injection Treatment
- Lidocaine (0.5%) is a fast and long-acting local anesthetic.
- Bupivacaine (0.25%) is lipophilic and is preferably used as a long-term anesthetic. When used at concentrations of up to 0.25%, it results in long-lasting analgesia with motor activity remaining largely unaffected.
- Ropivacaine (2mg/mL) was the first local anesthetic tested where clear, unambiguous results were obtained. Its blocking behavior demonstrates an advantageous weighting of sensory to motor effect.
Steroids are also infiltrated initially and during further treatment with local injections, as part of orthopedic pain therapy. The treatment focuses on the concomitant inflammatory reaction in the nociceptors and the area surrounding the afferent fibers. Steroids neutralize the pain-evoking prostaglandins and leukotrienes (Wehling 1993). For this reason, they have a local analgesic effect in addition to their anti-inflammatory action. The use of steroids with a high receptor affinity, such as triamcinolone, is preferable. A sufficiently high concentration of active ingredients in the immediate proximity of irritated structures is needed to ensure an effective pharmacodynamic interaction of steroids with the circumscribed inflammatory processes. The use of general medication such as orally administered steroids is therefore not a part of orthopedic pain therapy and is used only in exceptional cases. The concentration of steroid at the source of pain should be maintained for several weeks. At the same time, the systemic perfusion of glucocorticoids should be kept to a minimum in order to limit the pharmacodynamic loading on the entire organism. These guidelines can best be followed by using glucocorticoid depot preparations in the form of crystal suspensions. We therefore mainly use triamcinolone diacetate and triamcinolone acetonite when treating acute and chronic radiculopathies. Our research (Barth et al. 1990) has demonstrated that local administration of 5–10 mg of these steroids can saturate all steroid receptors in the surrounding tissues. Significant side effects (e. g., a sustained suppression of the body’s own cortisol production) are not to be expected when the steroid is administered in this form 1–3 times as part of a treatment cycle for a pain syndrome. Allergic reactions to carrier substances in steroids and local anesthetics are, however, to be anticipated when using all types of medication (see Chapter 10).
Therapeutic local injections are administered to painful muscle and tendon insertions as well as to different locations in the vertebral motor segments. The indications and techniques for individual injections can be found in the atlas section of this book.
Interleukin-1 Receptor Antagonist Protein (IL-1Ra)
As early as the 1920s, in connection with research into tuberculosis, it was already being speculated that special proteins act as messengers in “cell communication” (Zinsser and Tamiya 1926). Since then, a succession of new proteins has been characterized and an increasingly complex network of messengers has been discovered, initially with inconsistent nomenclature. In 1991 all mediators were for the first time grouped together under the term cytokines, and a systematic naming system was introduced (Klein 1991).
Interleukin 1 (IL-1) was first described in 1940, under the name “endogenous pyrogen.” It was subsequently found that this protein can be detected in all cells of the body, and that IL-1 receptors exist (Dower et al. 1984). Later, a naturally occurring antagonist was discovered (Liao et al. 1984) and was named anti-interleukin 1 or, more correctly, the interleukin 1 receptor antagonist protein, abbreviated to IRAP or IL-1Ra.
When the biological actions of IL-1 were studied, it was found to participate in the genesis and maintenance of acute and chronic inflammation as well as in the destruction of tissue. The nerve root, along with the articular cartilage, is an important target tissue in this context. More precise studies and experiments were able to demonstrate that a disproportionate ratio between agonist and antagonist seemed to govern certain disorders (Lennard 1995). Conditions where the proportion of IL-1 to IL-1Ra plays a central role include osteoarthrosis; intervertebral disk pathologies and nerve irritation syndromes that clinically present as lumbago or as lumboischialgia; and rheumatoid arthritis, especially the destruction of cartilage.
IL-1Ra is the only naturally occurring antagonist so far discovered within the cytokine family (Arend et al. 1989). As it acts by binding to the IL-1 receptor, the competitive binding of receptors seems to play a decisive role in pathology. An excess of IL-1Ra has to be present to repress IL-1 enough to antagonize its biological actions (Arend et al. 1990, Seckinger et al. 1990). This is the starting point when looking at the direction treatment should take. In certain disorders, such as osteoarthrosis and rheumatoid arthritis, there is a surplus of IL-1 relative to the levels of IL-1Ra. For this reason efforts are being made to administer IL-1 receptor antagonists exogenously in order to neutralize the action of IL-1.
The Role of IL-1 in Relation to Neural Structures
By the 1990 s it was already known that that cytokines, especially IL-1 acting as the primary mediator, could be responsible for neurological deficits and the development of pain. In the case of lumbago or lumboischialgia, mediators, primarily IL-1, are produced in the small vertebral joints or the intervertebral disk. These mediators are able to reach the area immediately surrounding the nerve roots and initiate pathological processes in the form of nerve inflammation and deterioration of function (Wehling 1991, Wehling et al. 1996). This knowledge resulted in the use of IL-1 receptor antagonists in the causal treatment of these symptoms.
Causal Therapy Using the IL-1Ra/Orthokine/EOT Technique
The Orthokine technique and the more recent forms of the so-called EOT technique have made available for the first time an autologous IL-1Ra that acts causally in the break-down process of joint wear or the inflammatory process at the nerve root.
It is known that IL-1 and the naturally occurring IL-1Ra can be found in all cells of the body, including certain blood cells and in particular the monocytes (Lennard 1995, Dinarello 1991). It has also been proven that some substances, superficial structures, and materials activate the production and release of IL-1Ra (Arend 1991 a, b). The Orthokine technique takes advantage of this fact by extracting venous blood from the patient with a specially developed syringe. The Orthokine syringe or EOT syringe contains glass beads which are made in such a way that their surface structure activates the monocytes to produce more IL-1Ra. The IL-1Ra-enriched protein concentrate can be separated following a specific incubation period at 37 °C, and extracted from the syringe without the addition of additives. The direct administration of active ingredient into the affected nerve root allows the ingredients to reach the affected area. The local enrichment of the autologous IL-1Ra at the inflamed nerve root causes an anti-inflammatory and anti-edemic effect by means of the occupation of receptors, thus switching off the action of IL-1.
The Orthokine method involves the physician taking approximately 60 mL of blood using the Orthokine syringe. The syringe is placed in an incubator and sent to the sterile laboratory at the manufacturing company Orthogen AG. The blood is serologically examined there, and the IL-1Ra-enriched serum is extracted and frozen. The treating physician receives at least six ampules of serum within about a week, following clearance from the laboratory.
The more recent EOT method is based on the same principles. In this method, however, treating physicians can manufacture the IL-1Ra-enriched serum themselves in a simple procedure using the EOT blood collection system. The special 5 mL Luerlock syringes used to take blood produce enough serum for injection (~2–4mL). Physicians can therefore decide themselves how many ampules are required, depending on the presenting disorder. The serum is available for injection after 6–9 hours of incubation; the waiting period of several days is no longer necessary. Serological testing for HIV, hepatitis B and C, and syphilis should be carried out before blood is collected (Figs. 4.10–4.15).