
Vertigo
Brian Chinnock
Vertigo, along with dizziness and other balance disorders, affects up to 70% of people during their lifetime. Vertigo is often categorized into peripheral and central causes. Peripheral disorders may cause considerable symptomatology but are rarely life-threatening, whereas those of central etiology may have significant morbidity and mortality.
PERIPHERAL AND CENTRAL VESTIBULAR ANATOMY
Maintenance of balance and coordination of eye movements is managed by a complex interaction of the vestibular system, the cerebellum, the brainstem, the ocular muscles, the visual cortex, and their interconnecting pathways. The peripheral vestibular system consists of three semicircular canals and two otolithic organs, the utricle, and saccule (Fig. 70.1A). Together they respond to head movement in all directions. Each canal is filled with endolymph, and has a dilated end called the ampulla which has the sail-like cupula. With head movement, the vestibular hair cells embedded in the cupula are stimulated and send input to the vestibular nerve (vestibular branch of CN VIII). Each semicircular canal is paired, and on head movement, one will give an excitatory input to the vestibular nerve, whereas the other will give an equal inhibitory input. With a unilateral peripheral vestibular deficit, the affected semicircular canal will not send in an inhibitory input to the vestibular nuclei, thus giving sensation of movement even if the head is still.
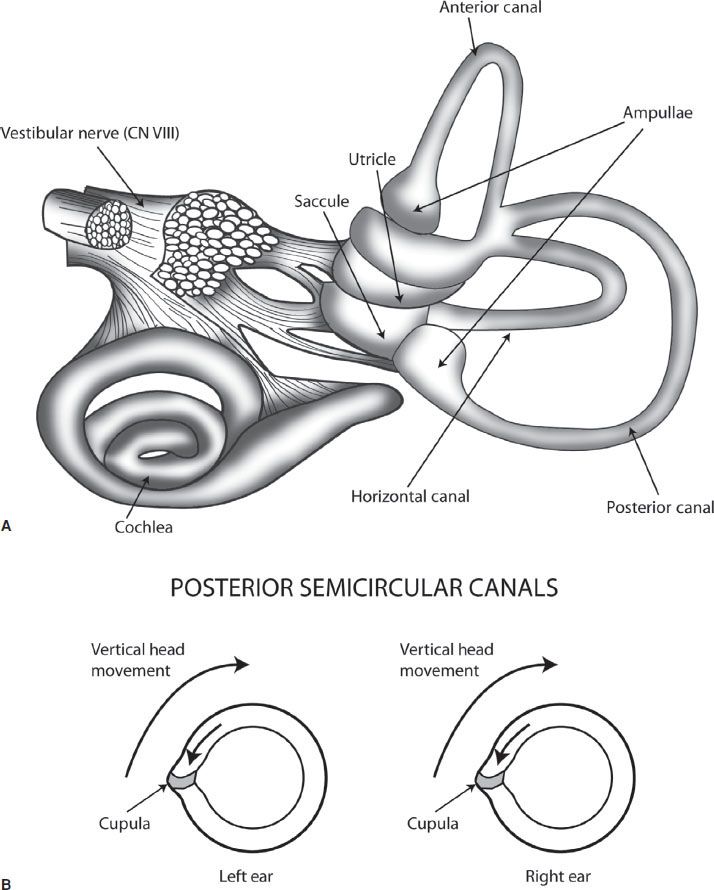
FIGURE 70.1 A: demonstrates the organs of the peripheral vestibular system. B: demonstrates the paired posterior semicircular canals, with vertical head movement causing the more static endolymph inside the canal to cause pressure against the cupula, which stimulates the vestibular nerve.
The central vestibular system receives input through the vestibular nerve to the vestibular nuclei located in the brainstem. From the vestibular nuclei, there are neuronal connections through the medial longitudinal fasciculus to the ocular muscles. This pathway is responsible for the vestibuloocular reflex (VOR), which does not involve the cerebellum, and is tested with the head impulse test (HIT) (see Figure 70.3). The cerebellum is the main organ of the central vestibular system, coordinating vestibular, visual, and somatosensory input to help fine-tune motor activity. The cerebellum is composed of the lateral hemispheres and the midline vermis. The lateral hemispheres are responsible for limb movement, whereas the vermis is responsible for truncal motion. The cerebellum receives its blood supply from the posterior-inferior cerebellar artery (PICA), the anterior-inferior cerebellar artery (AICA), and the superior cerebellar artery (SCA). All originate from the vertebrobasilar system. Importantly, the cerebellum is supplied by the distal branches of each of these arteries, with the proximal parts supplying the lateral brainstem. For this reason, it is common for cerebellar CVA to also present with brainstem findings.
PERIPHERAL VERSUS CENTRAL VERTIGO
While there are many causes of vertigo, there are four primary diagnoses of significance to the emergency physician evaluating a patient with acute vertigo: (1) benign paroxysmal positional vertigo (BPPV), (2) vestibular neuronitis (VN), (3) migrainous vertigo, and (4) cerebellar/brainstem ischemia (CBI). The discussion will focus on these four diagnoses as they are the most common and important. CBI, which includes cerebellar/brainstem transient ischemic attack/cerebrovascular accident (TIA/CVA) or vertebrobasilar insufficiency, only represents 1% to 3% of vertigo patients, but has a mortality rate of up to 40% (1). It is thus the most important differential diagnosis for any vertigo patient. Other causes of peripheral and central vertigo are listed in Table 70.1.
TABLE 70.1
Less Common Causes of Peripheral and Central Vertigo
BPPV: This is the most common cause of vertigo, representing up to 50% of cases. It has a cumulative incidence of nearly 10% by the age of 80. It is caused by otoconia, calcium carbonate crystals present in the utricle, that become dislodged and migrate into the semicircular canal. The presence of otoconia in the endolymph of one ear will cause increased excitatory input to the vestibular nuclei compared to the unaffected ear on head movement, causing vertigo. BPPV is characterized by intermittent episodes of sudden severe vertigo precipitated by head movement, commonly with nausea and vomiting, which usually last for less than 1 to 2 minutes. When the head stops moving, the vertigo will abate as the movement of the otoconia is the only source of abnormal input; the rest of the vestibular structures are fully functional. As the posterior canal is the most dependent of the semicircular canals and the most likely location for otoconia to settle by gravity (see Fig. 70.1A), 85% of BPPV is caused by posterior canal otoconia. While BPPV is commonly idiopathic, it may also be due to otoconia dislodged due to minor trauma or whiplash injuries, or as a residual effect of Ménière disease.
VN: VN is caused by a selective inflammation of the vestibular nerve, likely due to a post viral cause. Concomitant involvement of the cochlear nerve is termed “labyrinthitis,” and has the same physical examination findings as VN, in addition to hearing loss. Unilateral vestibular nerve involvement will give unequal input to the vestibular nuclei, and cause vertigo. Unlike BPPV, though, keeping the head completely still will not significantly affect the vertigo, as the defect is in the nerve itself, not the canals which give output based on movement. Resolution of symptoms typically takes weeks as recovery is dependent on the vestibular nerve regenerating, and central vestibular compensation mechanisms to develop, as the nerve regeneration is typically incomplete.
Migrainous vertigo: In neurootology clinics, this is the most common cause for referral. Criteria have been developed for the diagnosis of migrainous vertigo and are listed in Table 70.2. Despite these criteria, migrainous vertigo is a diagnosis of exclusion and can be the most difficult diagnosis to make as the vertigo can have either peripheral or central features (2). In addition, the quality and duration of vertigo can range from the mild, indolent quality of CBI which lasts for days, to the severe quality of BPPV or VN, lasting for minutes or over a day, respectively.
TABLE 70.2
Neuhauser Criteria for Migrainous Vertigo

CLINICAL PRESENTATION
A good history is extremely important in ruling out CBI in the vertigo patient. In one study, patients with isolated dizziness and no findings suggestive of CVA had a <1% incidence of CVA (3). Risk factors for CBI are the same as for other types of CVA: previous CVA, diabetes, hypertension, advanced age, atrial fibrillation, hypercholesterolemia. In a case series of patients with vertigo who had a final diagnosis of cerebellar CVA, and who had negative initial diffusion-weighted MRI (DW-MRI), the mean age was 72 years old and all had significant risk factors for CVA (4).
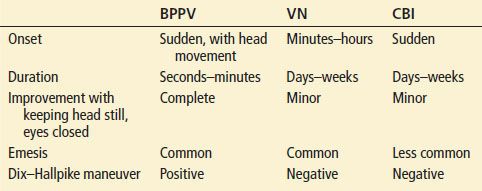
BPPV is usually easy to differentiate from VN and CBI by its sudden onset, short duration, and complete resolution of symptoms in between episodes (Table 70.3). Vertigo that is associated with particular head positioning is almost always due to BPPV (Table 70.3). However, rarely, a patient may have central positional vertigo due to a very localized infarction of CN VIII at the brainstem or of the vestibular nucleus. Either of these may cause a positional vertigo without other neurologic findings. The Dix–Hallpike maneuver (described below) may be particularly helpful in differentiating in this circumstance. Patients with migrainous vertigo usually have a previous history of migraines, with new vestibular symptoms which may or may not occur at the same time as the migraine.
TABLE 70.3
Features of BPPV in Comparison with VN and CBI
DIFFERENTIAL DIAGNOSIS
Traditional teaching emphasizes the differentiation of vertigo from presyncope, disequilibrium, or nonspecific dizziness. However, this approach may be problematic. A large ED-based study found that the incidence of CBI was similar in patients with vertigo versus nonvertiginous dizziness (3). Another ED study found that patients’ descriptions of dizziness were unclear, imprecise, and unreliable (9). Therefore the many causes of vertigo should be at least considered in patients without a clear description of vertigo.
ED EVALUATION
Physical Examination
Standard neurologic examination: All the standard elements of a neurologic examination should be completed, as the presence of any abnormalities can suggest CBI as the cause. Finger–nose testing for dysmetria by itself is not an adequate test of cerebellar function, since it tests only lateral hemispheric function. Large cerebellar vermal CVAs can have a completely normal finger–nose test. Testing of gait is extremely important as patients with peripheral vestibular disorders, while often having significant symptomatology trying to walk, will still be able to walk. Inability to walk (or sit or stand), strongly suggests CBI.
Nystagmus testing should be done by having the patient follow the examiner’s finger in all horizontal and vertical directions. It is also important to hold the finger for 10 to 15 seconds at eccentric vertical and horizontal gaze positions (testing for gaze-evoked nystagmus [GEN]), and then allowing the eyes to return to normal position (testing for rebound nystagmus). When doing this, it is important to not test at the very extremes of gaze as this can evoke a sustained nystagmus even in normal patients. In VN, the nystagmus will be unidirectional, horizontal, with perhaps a slight rotatory component, suppressible by fixating on a target. CBI is more likely if the nystagmus is vertical, purely rotatory, or is not suppressible. Physiologic end-point nystagmus (EPN) occurs upon extreme lateral gaze, when a few beats of low-amplitude nystagmus occur before normal brainstem mechanisms allow the eyes to maintain the eccentric gaze without further nystagmus.
GEN occurs on far eccentric gaze, with sustained, large-amplitude nystagmus. This is caused by an abnormality in the cerebellum or cerebellar pathways. A small lesion of CBI will only manifest at very extremes of gaze. A larger lesion can appear as soon as the eyes deviate from straight-ahead. A vertical GEN, in particular, is diagnostic of cerebellar or brainstem dysfunction. With VN, as the eyes move eccentrically in the direction of the fast phase of nystagmus, the nystagmus will increase until it eventually suppresses with fixation, whereas fixation is unlikely to suppress with CBI.
Rebound nystagmus occurs after holding the eccentric gaze position for 10 to 15 seconds, then returning to the primary gaze position. Nystagmus with the fast phase in the opposite direction of the preceding eccentric gaze indicates CBI. Reversible nystagmus occurs when the direction of the fast phase changes when looking side to side, and is indicative of CBI.
Smooth pursuit is assessed by watching eye movements while performing the nystagmus assessment. It is normal for patients to have slight saccades (fast, jerky eye movements) interrupting smooth pursuit during downward gaze, but should otherwise be smooth without saccades. Significant asymmetry of saccadic motion between each eye is suggestive of a structural brainstem/cerebellar lesion. Saccades due to slow eye movements (eyes have to make saccades to catch up with pursuit) can also be seen with medication/drug usage (alcohol, benzodiazepines).
Skew deviation is tested by asking the patient to fixate on the examiner’s nose, covering one eye, then uncovering that eye while simultaneously covering the other, alternating simultaneous cover and uncover every 1 to 2 seconds. Normally, there is little or no eye movement to regain fixation on the examiner’s nose. With CBI, fixation is not maintained and the eyes make a movement, usually vertical, to regain fixation. For example, as the left eye is uncovered it makes a vertical downward movement to regain fixation, and then, as the right eye is uncovered while the left eye is covered, the right eye makes a vertical upward movement to regain fixation. While not sensitive for CBI, the presence of skew deviation is very specific for CBI.
Dix–Hallpike: The Dix–Hallpike test should be performed if BPPV is a diagnostic consideration. The Dix–Hallpike test positions the head so that otoconia in the posterior semicircular canal move to cause maximal stimulation, with nystagmus and vertigo (Fig. 70.2, panels S and 1). The maneuver has an approximately 50% to 88% sensitivity in detecting BPPV (5).
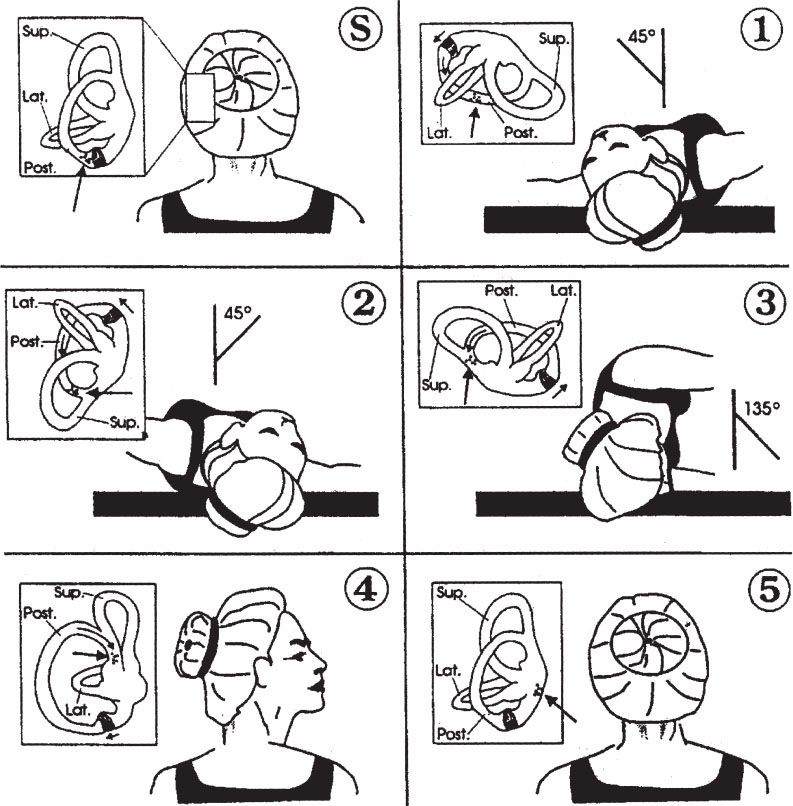
FIGURE 70.2 Dix–Hallpike test and Epley maneuver. Positioning sequence for left posterior semicircular canal as viewed by operator (behind patient). Small boxes show the labyrinth and migration of particles (large arrow). (S) and (1) represent a Dix–Hallpike test of the left posterior semicircular canal, while the entire sequence demonstrates the Epley maneuver. (S) Start-–patient seated. 1: Place head over end of table, 45 degrees to the left. For Dix–Hallpike test, with a positive test, there will be a short latency period, followed by an upward and torsional nystagmus and vertigo, which fatigue in about 15 to 30 seconds. When the nystagmus fatigues, the patient is returned to the sitting position, at which point the vertigo and nystagmus return briefly, the nystagmus now in the opposite direction. 2: Keeping head tilted downward, rotate to 45 degrees right. 3: Rotate head and body until facing downward 135 degrees from supine. 4: Keeping head turned right, bring patient to sitting position. 5: Turn head forward, chin down to 20 degrees. Pause at each position until induced nystagmus approaches termination, or for approximately 30 seconds if no nystagmus. If able, keep repeating entire series (1 to 5) until no nystagmus in any position.









