CHAPTER 20 Ventricular and Supraventricular Arrhythmias in Acute Myocardial Infarction
CARDIAC RHYTHM abnormalities occur in 72% to 95% of patients with acute myocardial infarction (MI) (Table 20-1).1–3 Because arrhythmias are more likely to occur early, and frequently occur before hospitalization, some cardiologists have suggested that the true incidence of arrhythmias associated with acute MI may be 100%.4 Arrhythmias associated with acute MI may occur because of re-entry, abnormal conduction or automaticity, and triggered activity. Several factors, including myocardial ischemia, infarct size, impaired hemodynamics, electrolyte abnormalities, and abnormalities of autonomic nervous system control, may influence these mechanisms and aggravate or cause arrhythmias.2,5,6
Table 20–1 Incidence of Arrhythmias in Acute Myocardial Infarction
| Arrhythmia | Incidence (%) |
|---|---|
| Sinus bradycardia | 10-55 |
| First-degree AV block | 4-15 |
| Second-degree AV block | |
| Mobitz type I | 4-10 |
| Multilevel AV block | 2 |
| Mobitz type II | Rare |
| Third-degree or complete AV block | |
| Inferior infarction | 12-17 |
| Anterior infarction | 5 |
| Asystole | 1-10 |
| Sinus tachycardia | 30 |
| Premature atrial contractions | 54 |
| Supraventricular tachycardia | <5 |
| Atrial fibrillation | 9-20 |
| Atrial flutter | 1-2 |
| Premature ventricular contractions | 90-100 |
| Accelerated idioventricular rhythm | 8-20 |
| Ventricular tachycardia | 10-40 |
| Ventricular fibrillation | 4-18 |
AV, atrioventricular.
Supraventricular Arrhythmias
Sinus Tachycardia
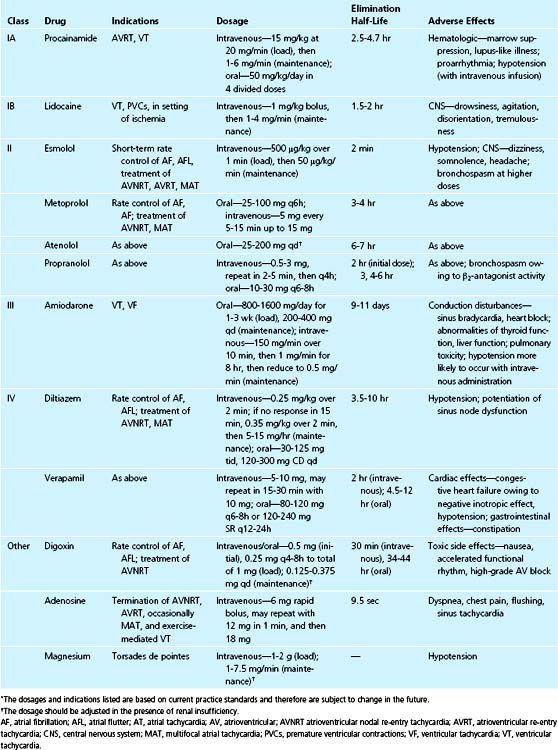
Sinus tachycardia is present in approximately 30% of patients with acute MI (see Table 20-1).7 This arrhythmia usually represents an increase in sympathetic activity and is more commonly seen in patients with anterior wall MI. Sinus tachycardia that persists beyond the initial 4 hours suggests that other causes of tachycardia may be present. Sinus tachycardia may be an early manifestation of heart failure, and in this setting is a poor prognostic sign. An underlying cause of the tachycardia should be determined (e.g., fever, pericarditis, pain, heart failure, anemia) and treated in an attempt to decrease myocardial oxygen demand. Specific therapy to slow the heart rate in the setting of anxiety and increased sympathetic activity includes administration of a β blocker (Table 20-2).
Atrial Arrhythmias
Atrial arrhythmias occur in 20% to 54% of patients with acute MI.5–11 Several studies have shown that patients with inferior MI are more likely to develop atrial arrhythmias early in their hospital course, but that patients with anterior MI tend to manifest atrial arrhythmias 12 hours to 4 days after MI.12,13 Several factors have been implicated in the pathogenesis of atrial arrhythmias, including atrial distention owing to underlying left or right ventricular dysfunction, pericarditis, and atrial infarction.
In a small series of patients with documented inferior MI, Rechavia and colleagues12 found that there was a significantly higher occurrence of premature atrial contractions and atrial fibrillation in patients with right ventricular dysfunction. It is unclear whether right atrial distention resulting from right ventricular dysfunction caused the premature atrial contractions and atrial fibrillation, or if there was associated right atrial infarction. Studies showing a higher incidence of atrial fibrillation in patients with atrial infarction and in patients with some extent of atrial infarction in right ventricular MI14 suggest that both factors may play a role.15 Atrial arrhythmias that occur late in the hospital course have been attributed to underlying left ventricular dysfunction. In these patients, treatment of congestive heart failure may prevent recurrence.12,16
Premature Atrial Contractions
Premature atrial contractions are the most common atrial arrhythmias, occurring in 54% of patients with acute MI (see Table 20-1).8,11 These beats may result from heightened sympathetic activity owing to pain or anxiety, or they may result from atrial distention, pericarditis, atrial infarction, or atrial ischemia. Although premature atrial contractions may precipitate other atrial arrhythmias, they are usually asymptomatic and are of no hemodynamic significance. Because premature atrial contractions are not associated with increased mortality, medical therapy to suppress these beats is not indicated.11
Paroxysmal Supraventricular Tachycardia
Paroxysmal atrial tachycardia and other re-entrant supraventricular tachycardias occur in less than 5% of patients (see Table 20-1).12,17 Most of these arrhythmias are transient, but the rapid ventricular rate is frequently associated with symptoms. Management of tachycardia should be directed at control of the ventricular rate. This control can be achieved promptly with intravenous administration of a β blocker or calcium channel blocker (see Table 20-2). These drugs or adenosine may terminate the tachycardia if the atrioventricular node is an integral part of the re-entry circuit. Adenosine may also terminate atrial tachycardia; however, it has the potential to result in atrial fibrillation.18 Caution should be used when administering any agent if symptoms of hypotension and congestive heart failure are present.
In patients with hemodynamic instability, synchronized DC cardioversion is the safest and the most expedient method to terminate the tachycardia. Treatment of underlying heart failure may prevent recurrences.12
Atrial Fibrillation and Flutter
Atrial fibrillation occurs in 9% to 20% of patients and is associated frequently with atrial infarction (see Table 20-1).8,12,14,19–21 Atrial flutter is uncommon in MI, occurring in only 1% to 2% of patients.11,12 There is no association between the occurrence of atrial or supraventricular arrhythmias and outcome.22–24 Earlier studies showed an increased mortality associated with supraventricular arrhythmias in the setting of acute MI.15,24,25 A later study designed to determine the prognostic value of supraventricular arrhythmias in the late phase of MI (13 to 19 days) found, however, that left ventricular ejection fraction was the only independent predictor of mortality.11 Serrano and coworkers12 determined the short-term and long-term outcomes of patients with supraventricular arrhythmias after MI. Although patients with arrhythmias during the late phase of MI (12 hours to 4 days) had significantly higher mortality rates at 1 month and 47 months, the presence of supraventricular arrhythmias was not an independent predictor of mortality. The increased mortality was not related to left ventricular function, but correlated with the presence of more extensive coronary artery disease.
Patients with atrial flutter typically have 2:1 conduction, and control of the ventricular rate may be difficult. DC cardioversion or pace termination via temporary atrial transvenous or epicardial wires can be performed to terminate the tachycardia. Recurrent episodes of atrial fibrillation or flutter may be prevented with administration of amiodarone. Patients with recurrent atrial fibrillation who meet current guidelines for anticoagulation should be started on warfarin therapy.26 Clopidogrel (Plavix) and aspirin do not prevent or reduce the risk of thromboembolism from atrial fibrillation.27
Ventricular Arrhythmias
Ventricular tachyarrhythmias are potentially the most dangerous arrhythmias associated with acute MI, occurring in 10% to 50% of patients.1,28 These arrhythmias are observed more frequently soon after the onset of MI. In experimental animals, factors that determine the occurrence of ventricular tachyarrhythmias include the size of the ischemic area,29 stress,30 preconditioning,31,32 increased heart rate,33,34 and autonomic nervous system influences.35,36 The occurrence of more than one factor may increase the risk for arrhythmias. Ischemia and sympathetic stimulation are more arrhythmogenic than either factor alone.37,38
Considerable evidence indicates that factors that influence arrhythmias in experimental models also influence the occurrence of ventricular tachyarrhythmias in humans. The incidence of ventricular tachyarrhythmias is related to infarct size and the presence of heart failure.36,39,40 Patients with an absolute increase in sympathoadrenal stimulation or decreased vagal efferent activity are at increased risk for sudden cardiac death and ventricular tachyarrhythmias.41–44 Acute MI causes several changes in the autonomic nervous system that may facilitate initiation of ventricular arrhythmias. Activation of cardiac mechanoreceptors and cardiopulmonary and carotid baroreceptors results in increased circulating catecholamine levels and increased efferent sympathetic activity. Ischemic damage to cardiac adrenergic neurons results in the release of catecholamines. Transmural MI denervates viable myocardium distal to the infarct site.45 Ischemic and denervated viable myocardium are hypersensitive to circulating catecholamines.44 Sympathetic stimulation also enhances automaticity in Purkinje fibers. These effects result in inhomogeneities of repolarization and enhance automaticity.
Electrolyte abnormalities, including hypokalemia and hypomagnesemia, are potentially correctable causes of ventricular arrhythmias associated with MI. Hypokalemia is an independent risk factor for ventricular arrhythmias early in MI.46 Hypomagnesemia often accompanies hypokalemia and may result in polymorphic ventricular tachycardia (VT). The use of thrombolytic drugs to treat patients with acute MI has renewed interest in arrhythmias associated with coronary reperfusion. These arrhythmias were observed in 1881, when ventricular fibrillation (VF) was seen within seconds after restoration of coronary flow.47 Coronary reperfusion may cause isolated premature ventricular contractions, accelerated idioventricular rhythm (AIVR), VT, or VF, presumably by enhancing automaticity of ischemic myocardium.48 The severity of the arrhythmia induced by reperfusion is related to the duration of myocardial ischemia.
The presence of certain antiarrhythmic drugs at the time of MI may facilitate initiation or maintenance of ventricular arrhythmias. This proarrhythmic effect is thought to occur because of changes in the electrophysiologic action of the drugs in the setting of myocardial ischemia. In a canine model, flecainide facilitated induction and sustenance of VT, particularly at shorter cycle lengths.49 The effect seemed to result from slowing of conduction in ischemic myocardium. Although less well studied, ischemia-induced proarrhythmia may occur with type IA or type III antiarrhythmic drugs.
Ventricular Premature Beats
Ventricular premature beats occur in almost all patients with acute MI50 (see Table 20-1), and are rarely a cause of myocardial ischemia or systemic hypotension. Previously, these beats were thought to be important because of their potential to trigger VT or VF. Early studies suggested that the frequency and timing of ventricular premature beats was associated with the risk for life-threatening arrhythmias.51 Early ventricular premature beats (R on T phenomenon) were thought to initiate VF because they depolarized the ventricle during the vulnerable period when ventricular refractoriness was inhomogeneous, and re-entry was more likely to occur. Data from animal models and human studies indicate, however, that frequent and complex ectopy is neither a sensitive nor a specific predictor for development of sustained ventricular tachyarrhythmias early after MI.28,52–57
The incidence of frequent ventricular premature beats and the R on T phenomenon is similar between patients who develop VF and patients who do not develop VF.28 Studies of animals and humans have shown that most VT episodes58 and 41% to 45% of VF episodes52,53 are initiated by late-coupled ventricular premature beats (after the T wave), suggesting that early beats are not required to initiate the arrhythmia. VF occurs in the absence of preceding ventricular ectopy in 40% to 83% of patients.52,53
Ventricular Tachycardia
VT appears on the surface 12-lead electrocardiogram (ECG) as a wide QRS complex tachycardia. Although several criteria and algorithms have been proposed to differentiate this arrhythmia from supraventricular tachycardia with aberrant ventricular conduction, the diagnosis sometimes remains uncertain (Table 20-3).59,60 When a definitive diagnosis is impossible, a wide QRS complex tachycardia associated with acute MI should be considered ventricular in origin until proved otherwise.
Table 20–3 Electrocardiographic Criteria That Support the Diagnosis of Ventricular Tachycardia
| QRS complex width >0.14 sec |
| Left-axis deviation |
| Configuration of QRS complex |
| In RBBB: monophasic or biphasic complex in lead V1 |
| In LBBB: Q wave in lead V2 |
| Concordance in the precordial leads |
| AV dissociation∗ |
| Absence of an RS complex in the precordial leads∗ |
| If RS complex is present, an RS interval >0.10 sec∗ |
AV, atrioventricular; LBBB left bundle branch block; RBBB, right bundle branch block.
∗ 100% specific for ventricular tachycardia.
From Mackall JA, Buchler CM, Thames MD. The pharmacological approach to the management of the cardiac surgical patient. In Baue AE, (ed): Glenn’s Thoracic and Cardiovascular Surgery. East Norwalk, CT, Appleton & Lange, 1998, p 1613.
Additional measures may differentiate VT from supraventricular tachycardia with aberrant ventricular conduction. Vagal stimulation (with a Valsalva maneuver or carotid sinus massage) or administration of adenosine may transiently slow or terminate supraventricular tachycardia, but does not affect most VT.61 Intracardiac electrograms may be recorded from temporary pacemaker wires to show the relationship of atrial and ventricular activation. The presence of atrioventricular dissociation during a wide QRS complex tachycardia is almost always diagnostic of VT. Administration of lidocaine may be diagnostic and therapeutic because lidocaine has no activity in atrial tissue.
VT occurs in 10% to 40% of patients with acute MI (see Table 20-1).1,28 The timing of VT has important implications regarding the mechanism and prognosis of the arrhythmia. VT that occurs late in the course of MI (after 48 hours) is more common in patients with transmural infarction and left ventricular dysfunction.1,62 When it occurs late, is sustained, and results in hypotension, VT is likely to recur and is associated with increased in-hospital and long-term mortality rates.62–64 VT that occurs early is more likely to have reversible causes (e.g., ischemia, reperfusion, autonomic nervous system influences) and is less likely to recur.
Sustained monomorphic VT most often results from re-entry, usually within a single circuit.65 Patients with this arrhythmia, even when it occurs less than 48 hours after the onset of acute MI, should undergo a thorough evaluation to determine the risk for recurrence and the need for therapy. VT resulting from reperfusion often occurs within the first hour after administration of thrombolytic therapy, is often associated with a slower rate, and is less likely to recur.
Polymorphic VT differs from monomorphic VT in that the QRS complex morphology varies from beat to beat. This arrhythmia has been reported to occur in 0.7% to 2% of patients hospitalized for acute MI.66,67 Although the mechanism for the arrhythmia is unknown, polymorphic VT is not thought to be associated with a stable re-entrant circuit. When the arrhythmia occurs early (within 6.5 hours of the onset of symptoms), it is often associated with coronary reperfusion and a good prognosis.67 When it occurs late (2 to 13 days after acute MI), the arrhythmia is associated with recurrent ischemia and a poor prognosis.66