Fig. 25.1
Large central thrombi in the main bilateral pulmonary arteries (arrows) in a 72-year-old man who presented with submassive pulmonary embolism
Question
If the decision is made to administer thrombolytic therapy, how would this treatment alter the patient’s overall outcome? His survival?
Answer
Treatment with thrombolytic therapy would likely prevent hemodynamic collapse but would not affect his chances of survival.
In the PEITHO trial, 1005 patients with sub-massive pulmonary embolism, defined by right ventricular enlargement or dysfunction and myocardial injury as indicated by an elevated troponin I or troponin T, were randomized to receive IV heparin and placebo versus IV heparin and recombinant Tissue plasminogen activator (t-PA) [1]. The primary endpoint was a clinical composite of death or hemodynamic decompensation within 7 days after randomization. The investigators also assessed a safety endpoint of bleeding complications. The results of this study revealed that treatment with t-PA significantly reduced the primary endpoint of death or hemodynamic decompensation within 7 days after randomization (2.6 % versus 5.6 %, p = 0.02). The difference in the primary endpoint was driven by a reduction in hemodynamic collapse as mortality was not different between the two treatment groups. The benefit of a reduction in the primary outcome with t-PA treatment came with the expense of an increased risk of major bleeding events including extracranial bleeding (p < 0.001) and strokes (p = 0.003).
Standard Approach to Diagnosis and Management
Risk Factors
Pulmonary embolism is a consequence of thrombosis in the deeps veins of the lower extremities, pelvis, and less commonly, the upper extremities. The classic triad of risk factors for the development of deep venous thrombosis, known as Virchow’s triad, includes stasis of blood flow, hypercoagulability, and endothelial injury. Patients admitted to the intensive care unit frequently experience all of these risk factors and are therefore at a heightened risk for deep venous thrombosis (DVT) and, in turn, pulmonary embolism (PE). Specifically, mechanical ventilation, sedation, and the use of paralytic agents accentuate the immobility of critical illness and thereby contribute to stasis of blood flow. In addition, central lines inserted into the upper and/or lower extremity deep veins serve as a nidus for thrombosis secondary to local endothelium disruption. Central lines placed in the femoral and internal jugular veins are associated with a particularly high risk, and the likelihood of this complication increases with the duration of catheter placement [2, 3]. Finally, the clinical diagnosis that necessitates ICU admission can modify the risk of venous thromboembolism (VTE). For example, immobility secondary to infection was associated with a shorter duration until the onset of thromboembolism as compared to patients whose immobility was due to dementia (less than 4 weeks in 94.2 vs. 25.9 % of cases; p < 0.001) [4]. The clinical presentations of severe sepsis and septic shock are also associated with a high rate of VTE [5]. The mechanistic connection between infection and venous thrombosis is in part explained by inflammation-induced: (1) elaboration of tissue factor, (2) impairment of anticoagulant pathways, and (3) suppression of fibrinolysis secondary to the overproduction of plasminogen activator inhibitor-1 [6].
Epidemiology of VTE in the ICU
The presence of multiple risk factors for VTE in ICU patients confers a high risk of disease. Clinical studies demonstrate that both unfractionated heparin and low molecular weight heparin decrease the risk of DVT in the critical care unit [4, 7]. As a result of this evidence, current practice guidelines recommend the administration of thromboprophylaxis in the critical care setting. Therefore, it is not surprising that, in a multivariate analysis of risk factors for PE in a Tunisian ICU, the absence of pharmacologic prevention was identified as a significant predictor for PE [8]. However, even in the presence of prophylactic heparin treatment, the risk of VTE is significant. In the PROTECT study, the efficacy of low molecular weight heparin (LMWH) and unfractionated heparin (UFH) were compared in the prevention of DVT in critically ill patients [9]. Evidence for new DVT formation was determined by the performance of twice-weekly compression ultrasonography. Despite the administration of prophylactic anticoagulation, the rates of DVT were 5.1 % in patients treated with LMWH and 5.8 % in patients treated with UFH. The risk of DVT appears to be even greater in patients with acute decompensated COPD who undergo mechanical ventilation [10]. In this population, treatment with weight-adjusted LMWH was associated with a DVT incidence of 15.5 % while patients receiving placebo experienced a 28.2 % incidence of clot.
Although evidence indicates that the incidence of DVT in the ICU is high, it is less clear how often this complication results in pulmonary embolism. In the PROTECT trial, PE was evaluated when clinically indicated, and in patients treated with LMWH, there was a 1.2 % incidence of definite or probable PE as compared to a 2.1 % incidence in the group treated with UFH. While these incidences are low and reassuring, results from a 2012 study suggests that the diagnosis of PE may be frequently missed in the ICU [11]. In this investigation, 176 consecutive mechanically ventilated patients who required a CT scan for any indication underwent the standard imaging protocol for pulmonary embolism detection. The investigators discovered that the incidence of PE was 18.7 %, and in 61 % of these patients, there was no clinical suspicion of disease. Importantly, the study protocol called for the performance of lower extremity compression ultrasonography in all patients within 48 h of their CT scan, and only 33 % of individuals with a PE were found to have a concurrent DVT. Collectively, these studies demonstrate that both DVT and PE are relatively common in the ICU despite thromboprophylaxis. Perhaps even more concerning is the observation that the majority of patients diagnosed with a PE lacked clinical features of the disease.
Clinical Presentation
The clinical presentation in the accompanying case scenario is highly suggestive of pulmonary embolism. However, in the ambulatory population, the clinical features of venous thromboembolic disease are largely non-specific. The lack of specificity in both symptoms and signs of pulmonary embolism was highlighted in PIOPED II, a study in which patients with suspected PE were enrolled to evaluate the predictive value of Computed-tomography pulmonary angiography (CT-PA) [12]. As part of this study, the frequency of symptoms and signs in patients with confirmed PE were compared to enrolled patients who ultimately ruled out for thromboembolism. Surprisingly, this investigation revealed that the presentation of hemoptysis or pleuritic chest pain was more common in PE-negative patients than in the PE-positive group (56 vs 44 %, p < 0.01). Furthermore, there was no difference between the two groups in the frequency of presenting with circulatory collapse. The presence of uncomplicated dyspnea (i.e. dyspnea without accompanying symptoms of chest pain, hemoptysis or circulatory collapse) was more common in patients diagnosed with PE than the PE-negative group but the overall percentages were similar (36 % versus 26 %, P < 0.01). With respect to specific symptoms or signs, the complaint or clinical detection of calf or thigh swelling or pain was most discerning for those who did versus those who did not have a PE.
In the critical care patient, co-morbid conditions that are associated with hypotension and hypoxemia make it even more challenging to identify patients with VTE. In a single center study of 4408 ICU patients, 87 (1.9 %) were diagnosed with pulmonary embolism [8]. Abnormalities in this group that led to the evaluation and diagnosis of PE included hypotension (57.5 %), positive SIRS criteria (72.4 %), respiratory distress requiring mechanical ventilation (81.6 %), and clinical manifestations of DVT (17.2 %). These derangements, perhaps with the exception of clinical manifestation of DVT, are not specific for PE and can be observed in other conditions that commonly lead to ICU admission including septic or hemorrhagic shock, congestive heart failure, and pneumonia.
The low sensitivity and specificity of symptoms and signs for VTE motivated the development of clinical prediction tools including the Wells Criteria (Table 25.1) and the Geneva Scoring System (Table 25.2) to aid clinicians in the evaluation of VTE. The Wells criteria either dichotomizes or trichotomizes patients into VTE risk categories [13]. This prediction tool has been well validated in the outpatient setting, but in hospitalized patients, it was found to perform less well for the diagnosis of DVT [14]. The Wells Criteria and the Geneva Score have not been explicitly evaluated in the critical care setting. However, based on the challenge of evaluating symptoms in the ICU patient who may be intubated, sedated, and/or delirious and in the presence of concurrent critical illnesses that commonly result in tachycardia and lower extremity edema, it is likely that the predictive value of this tool, like the inpatient setting, is weak in the critical care population. The limitation of these tools in the ICU setting is supported by the findings of Bahloul and colleagues who calculated both the Wells and Geneva scores in 87 patients who were diagnosed with PE in the critical care setting [8]. In this study, only 5 (5.7 %) patients had a high probability score using Wells Criteria and only 6 (6.9 %) patients were classified as high probability according to Geneva Score. Based on these findings, it is crucial to maintain a high index of suspicion for VTE as a potential etiology of subtle (and not so subtle) physiologic changes in an ICU population.
Table 25.1
Wells criteria for pulmonary embolism risk
Variable | Points |
|---|---|
Clinical signs and symptoms of DVT | 3 |
Alternative diagnosis less likely than PE | 3 |
Heart rate >100/min | 1.5 |
Immobilization (>3d) or surgery in the prior 4 wk | 1.5 |
Prior PE or DVT | 1.5 |
Hemoptysis | 1 |
Malignancy (receiving treatment, treated in last 6 mo, palliative) | 1 |
Score | Clinical probability | PE incidence |
|---|---|---|
Trichotomized score | ||
0–1 | Low | 0.2–7.0 % |
2–6 | Moderate | 12.4–26.6 % |
≥6 | High | 27.2–72.8 % |
Dichotomized score | ||
≤4 | Unlikely | 2.3–9.4 % |
≥4 | Likely | 27.6–51.6 % |
Table 25.2
Revised Geneva Score for pulmonary embolism risk
Variable | Points |
|---|---|
Risk factors | |
Age > 65 y | 1 |
Prior PE or DVT | 3 |
Surgery (under general anesthesia) or fracture of the lower limbs within 1 mo | 2 |
Active malignant condition (solid or hematologic, currently active or considered cured < 1 y) | 2 |
Symptoms | |
Unilateral lower-limb pain | 3 |
Hemoptysis | 2 |
Clinical signs | |
Heart rate | |
75–94 beats/min | 3 |
≥95 beats/min | 5 |
Pain on lower-limb deep venous palpation and unilateral edema | 4 |
Score | Clinical probability | PE incidence |
|---|---|---|
0–3 | Low | 5.0–12.0 % |
4–10 | Intermediate | 24.6–32.8 % |
≥11 | High | 61.0–83.4 % |
Pulmonary Embolism Severity
Patients with pulmonary embolism can be categorized into low, intermediate, and high risk groups. Stratifying individuals into risk categories can be useful in decisions about disposition and treatment. The presence of hemodynamic collapse, which results from an acute increase in pulmonary artery pressure and associated right heart failure, readily classifies patients into a high-risk group with an estimated mortality of 30–50 % and mandates a treatment strategy that includes vascular reperfusion (see section “Treatment”). Hemodynamic stability in the presence of right ventricular (RV) dysfunction defines an intermediate risk category. Evidence of RV dysfunction can be detected on imaging studies including echocardiography (Fig. 25.2) and CT-PA and with elevated biochemical markers such as brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), troponin-I, and troponin-T levels. Several systematic reviews reveal an increased risk of death in patients with pulmonary embolism who also have evidence of abnormal RV function by imaging or elevated biomarkers. For example, in a recent meta-analysis, CT-PA evidence of right heart dysfunction carried an odds ratio of death from PE of 7.4 (95 % confidence interval 1.4–39.5) [15]. A separate study found that either CT-PA or echocardiographic evidence of RV dysfunction was associated with an unadjusted risk ratio for death of 2.4 (95 % confidence interval 1.3–4.4) [16]. In this same systematic review, elevations in brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin also predicted an increased risk of death, but the threshold values for these biomarkers varied considerably between studies. Because of the higher rate of death, patients in this intermediate risk group may hypothetically benefit from aggressive treatment to reestablish vascular reperfusion (see section “Treatment”).
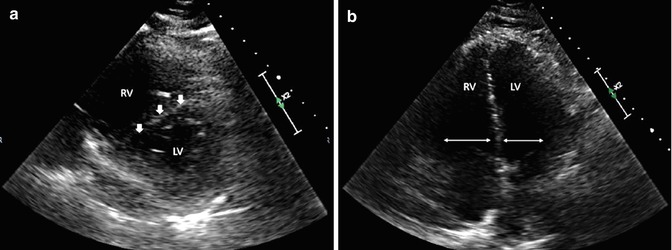

Fig. 25.2
(a) Right ventricular (RV) strain seen on parasternal short axis view with the intraventricular septum (arrows) bowing into the left ventricle (LV). The result is known as a “D-sign”. (b) Increased RV/LV ratio seen on apical four chamber view
Beyond categorizing patients based on the presence/absence of hemodynamic instability or RV dysfunction, several prognostic scoring systems have been developed to estimate the 30-day mortality in patients with PE. These tools primarily aid clinicians in their decisions about patient disposition. Individuals with low scores may be safely managed as outpatients while patients with high scores require hospitalization in an acute care bed or in the intensive care unit. The Pulmonary Embolism Severity Index (PESI) and the simplified PESI are examples of these risk predictor tools (Table 25.3). The PESI is comprised of 11 simple patient variables that are each independently associated with PE mortality [17]. The composite score assigns individuals to one of five risk categories. In class I and II, the lowest risk categories, 30-day mortality rates were between 0 and 3.5 % in the derivation and validation groups. In contrast, patients in the highest risk category (Class V) exhibited 30-day mortality rates ranging between 10.0 and 24.5 %. More recent data suggests that combining the PESI with echocardiographic and biomarker data can further refine risk stratification [18]. Whether these tools of risk prediction play any role in an ICU patient with VTE is unclear. However, for those individuals diagnosed with PE outside of the intensive care unit, it may be prudent to consider admission to a higher level of care such as a critical care unit if a patient is determined to be in the highest risk category for mortality.
Table 25.3
Pulmonary embolism severity index
Predictors | Points |
|---|---|
Demographics | |
Age, per year | Age in year |
Male sex | +10 |
Comorbid illnesses | |
Cancer | +30 |
Heart failure | +10 |
Chronic lung disease | +10 |
Clinical findings | |
Pulse ≥ 100/min | +20 |
Systolic blood pressure < 100 mmHg | +30 |
Respiratory rate ≥ 30/min | +20 |
Temperature < 36 °C | +20 |
Altered mental status | +60 |
Arterial oxygen saturation < 90 % | +20 |
Score | Risk class | 30-day mortality |
|---|---|---|
≤65 | I, very low | 0–1.6 % |
66–85 | II, low | 1.7–3.5 % |
86–105 | III, intermediate | 3.2–7.1 % |
106–125 | IV, high | 4.0–11.4 % |
>125 | V, very high | 10.0–24.5 % |
Diagnosis
With a few exceptions, the diagnostic approach to VTE in the ICU should not be fundamentally different than in the emergency department or on the general care wards. The initial step in evaluating an ambulatory patient (i.e. presenting to the emergency department or clinic) with suspected pulmonary embolism is to assess the clinical probability of disease. The implementation of a clinical probability tool such as Wells Criteria greatly facilitates this assessment. Those individuals determined to have a low likelihood of PE (Table 25.1) should be initially evaluated with a d-dimer. Patients with a high clinical likelihood of disease (or low clinical likelihood and a positive d-dimer) should undergo a diagnostic study for PE such as a ventilation-perfusion scan or a CT-PA. The latter imaging approach is often the preferred modality because it also allows for the detection of other abnormalities that might explain a patient’s clinical syndrome, and it can provide information about the status of the right ventricle. Based on the results of PIOPED I and PIOPED II, additional diagnostic studies such as pulmonary angiography should be entertained when the results of the CT-PA or ventilation/perfusion scan are discordant with the clinical probability of disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







