INTRODUCTION AND EPIDEMIOLOGY
Pulmonary embolism (PE) occurs when clotted blood enters the pulmonary arterial circulation. Most PEs result from deep vein thrombosis (DVT) in the legs, arms, or pelvis and occasionally from the jugular vein or inferior vena cava. The term venous thromboembolism (VTE) includes PE and DVT.
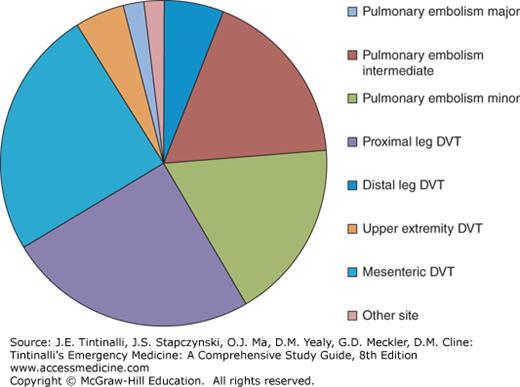
In the United States, approximately 200,000 people will have new or recurrent PE diagnosed each year, and twice that many will have DVT without confirmed PE.1 VTE collectively affects about 1 in 500 persons per year in North America, and about 1 in every 300 adult ED patients receive the diagnosis (Figure 56-1). The incidence of VTE increases with age, peaking at 1 in 100 per year at age 80. Based on autopsy data, PE is the second leading cause of sudden, unexpected, nontraumatic death in outpatients.2 The case fatality rate from PE depends on the hemodynamic severity of the PE, age, and comorbid conditions; the case fatality rate is 45% for PE with circulatory shock, but only about 4% to 5% of patients with PE have shock. In patients with hemodynamically stable PE who are less than 50 years old and without other comorbidities, the case fatality rate is 1%.3
FIGURE 56-1.
Distribution of the frequency of clots diagnosed by emergency physicians by severity and location. Major refers to pulmonary embolism (PE) with hypotension, intermediate is PE with right ventricular dysfunction, and minor is small PE without hemodynamic effect. Distal deep vein thrombosis (DVT) refers to calf vein and saphenous vein thrombosis, and other sites include inferior vena cava, pelvic, jugular, ovarian, cerebral, and retinal veins.
Morbidity from DVT includes PE and the postthrombotic syndrome. The latter is manifested as chronic leg swelling and pain and occurs in about 20% of all ED patients with proximal DVT.4 Both DVT and PE present across a spectrum of severity, with recognition of minor forms of the disease, including distal PE (called subsegmental) and distal DVT, usually in the calf or saphenous veins.5
PATHOPHYSIOLOGY
Blood clots occur when coagulation exceeds the removal by fibrinolysis. Thrombophilias are conditions that tip the balance of coagulation-fibrinolysis toward excessive clotting. Most guidelines categorize VTE as provoked (or secondary) or unprovoked (idiopathic).6 Provoked VTEs are acquired and often time-limited conditions, generally often following recent surgery, trauma, or any condition associated with limb or body immobility; active cancer is a VTE provoker that often persists. Other provoking factors generally include diseases or conditions that impede venous blood flow, infection, chronic disease, estrogen use, pregnancy or initial postpartum interval, and age >50 years (each year after 50 increases the risk). Unprovoked VTE patients have no known risk factors, suggesting an increased tendency to clot.
Most VTEs diagnosed in the ED are unprovoked.3,7 Patients with unprovoked VTE have a 15% chance of recurrence in the next year compared with 5% for those with a provoked episode. For this reason, those with unprovoked VTE usually receive longer treatment than patients with provoked VTE.8 Those with provoked VTE have a higher 1-year death rate, likely from the comorbid conditions (notably cancer).9
Venous thrombi that are large enough to cause clinically important PE can form in the popliteal, common femoral, superficial femoral, pelvic, axillary, jugular, and great veins. At least one third of patients with DVT have concomitant PE, even when the patient lacks symptoms of PE.10 Although 75% to 80% of hospitalized patients with PE have image-demonstrated DVT, only 40% of ambulatory ED patients with PE have concomitant DVT.11
Blood clots that form in the large veins of the legs, especially the femoral and iliofemoral veins, usually begin on the valves, leading to scarring and poor function of the venous valves. This causes pooling of venous blood in the legs, leading to varicose veins, pain, swelling, skin hyperpigmentation, and ulcers, known as the postthrombotic syndrome.4 With PE, the correlation between the degree of initial pulmonary vascular obstruction and clinical severity is weak, but patients without prior heart or lung disease generally begin to experience symptoms from PE when approximately 20% of lung vasculature becomes occluded.12 With larger clot burden, the pulmonary arterial pressure increases, leading to right ventricular dilation and myocardial damage, causing the release of troponin and B-type natriuretic peptide. Right ventricular dilation or injury, as evidenced on CT or echocardiography or suggested by elevated troponin or B-type natriuretic peptide, indicates right heart failure and increased risk of circulatory shock and death.13 The two principle mechanisms of death from PE appear to be abrupt near-total pulmonary artery occlusion that leads to pulseless electrical activity and asystole from ischemic effect on the His-Purkinje conduction system. Approximately one third of survivors of large PEs go on to have persistent right heart dysfunction and severe symptoms.14 Approximately 5% of patients with PE go on to experience chronic pulmonary vascular obstruction, leading to progressive damage to the lung vessels and right heart with disabling dyspnea and pulmonary hypertension, called chronic thromboembolic pulmonary hypertension.
Inherited thrombophilias increase risk of first-time VTE, although most of these patients are unaware of this condition until a clot occurs. The risk of recurrent VTE in those with a known thrombophilia is the same as in those who have had a prior unprovoked VTE absent that condition.15
With limb immobility, the risk increases depending on joint, as follows: elbow (least), shoulder, ankle, knee, and hip (most). Acute immobilization of the hip and knee in one leg with non–weight bearing causes the greatest risk for VTE, whereas immobilization of the wrist alone probably causes no risk. In addition to the presence of limb immobility, risk of VTE increases with whole-body immobility or neurologic immobility, but not by travel, even if >8 hours.16
Over half of patients with postoperative PE receive the diagnosis after hospital discharge, and the average time from surgery to PE diagnosis is >10 days. Risk increases with patient age, longer surgery, open surgery, and surgery in which thromboprophylaxis is not used. The highest-risk surgeries include abdominal surgery to remove cancer, joint replacement surgery, and surgery on the brain or spinal cord in the setting of neurologic deficits. However, the risk of recurrence after surgery-provoked VTE is generally lower than for unprovoked VTE, although the 4-year risk of recurrence ranges from 5% to 11% per year depending on the surgical procedure.17
Thrombogenic potential of cancer varies with host factors, tumor stage, and tumor type. In general, the more undifferentiated the cell type and the larger the tumor burden (especially distant metastasis), the higher is the risk. Cancers that are particularly thrombogenic include adenocarcinoma, glioblastoma, metastatic melanoma, lymphoma, and multiple myeloma.18 Pancreatic, stomach, ovarian, and renal cell cancers carry notoriously high risk.
Cancers with minimal risk of VTE include localized breast, cervical, prostate, and nonmelanomatous localized skin cancers such as squamous cell carcinoma and basal cell carcinoma not treated with chemotherapy. However, about 10% of patients with advanced-stage breast cancer or with breast cancer undergoing chemotherapy develop symptomatic VTE. VTE risk is high during the induction phase of chemotherapy, especially if treated with L-asparaginase and bolus fluorouracil or tamoxifen. Concomitant treatment with red blood cell growth factors such as erythropoietin increases risk of thrombosis, regardless of tumor type or stage.18 Similarly, multiple myeloma patients treated with lenalidomide or thalidomide are at high risk of VTE.19
A family history of VTE increases longitudinal risk of VTE,20 although this has no impact on outcomes.7 Although sex does not play a role in first-time VTE, men have more recurrent VTE.8
Table 56-1 presents a list of risk factors for VTE relevant to ED practice.
| Factor | Comment |
|---|---|
| Age | Risk becomes significant at 50 y and increases with each year of life until age 80 y. |
| Obesity | In the general population, VTE risk starts at BMI >35 kg/m2 and increases with increasing BMI. |
| Pregnancy and postpartum state | 70% of all peripartum PEs occur postpartum. Risk increases with trimester (but overall risk remains low throughout pregnancy). |
| Prior VTE | Highest risk of recurrence is for unprovoked VTE in men, particularly if d-dimer remains elevated |
| Solid cancers | Risk greatest with adenocarcinomas and metastatic disease. A history of remote, inactive cancer probably does not increase risk. |
| Hematologic cancers | Acute leukemias and myeloma confer the greatest risk, particularly when treated with L-asparaginase and the thalidomide derivatives. |
| Thrombophilias | Non-O blood type, lupus anticoagulant, shortened aPTT, factor V Leiden, and familial protein C and S and antithrombin deficiency have the strongest risk. |
| Recent surgery or major trauma | Risk increased with endotracheal intubation or epidural anesthesia and continues at least 4 weeks after exposure. Risk varies with type of surgery. |
| Immobility | Acute limb immobility of two contiguous joints confers the highest risk. |
| Bed rest | Becomes a risk factor at approximately 72 h. |
| Indwelling catheters | Cause approximately one half of arm deep venous thromboses. |
| Long-distance travel | Published data are controversial. In general, risk becomes significant after 6 h of continuous travel. |
| Smoking | A population risk factor, but not a factor that increases probability of VTE in the ED setting. May increase risk of other factors such as obesity. |
| Congestive heart failure | Related primarily to severity of systolic dysfunction. |
| Stroke | Risk greatest in first month after deficit. |
| Estrogen | Highest-risk period is in the first few months. All contraceptives containing estrogen increase risk of VTE including transdermal and transvaginal preparations. |
| Noninfectious inflammatory conditions | Examples are inflammatory bowel disease, lupus, and nephrotic syndrome. Risk of VTE increases roughly in proportion to severity of underlying disease. |
Although smoking causes conditions that increase risk (e.g., cancer) and acts synergistically with obesity and possibly oral contraceptive use, smoking is not an independent risk factor for VTE.7
CLINICAL FEATURES OF PULMONARY EMBOLISM
PE symptoms range from none to sudden death. Patients with similar comorbidities and clot burden may have drastically different clinical presentations. Table 56-2 lists factors that affect symptoms.
| Cofactor | Clinical Impact | Comment |
|---|---|---|
| Previously healthy and young age | Less severe signs and symptoms | One half of previously healthy patients with first-time PE have normal vital sign values at diagnosis. |
| Prior cardiopulmonary disease | Can either amplify or obscure history and findings | Most patients with PE complicating baseline cardiopulmonary disease describe dyspnea with PE as “worse than usual.” |
| Patient cognitive dysfunction | Causes the history to be less reliable | Approximately 20% of patients with PE missed by ED clinicians had baseline dementia. |
| Clot size and location | Affects severity of dyspnea, pain, and signs | Proximal clots cause ventilation–perfusion mismatch and dyspnea; distal clots cause infarction with pain. |
| Gradual loading of PE over time | Gradual onset of dyspnea on exertion and fatigue | Has symptom overlap with left ventricular dysfunction. Fewer than one half of patients with PE describe symptom onset as sudden. |
The hallmark of PE is dyspnea unexplained by auscultatory findings, ECG changes, or clear alternative diagnosis on chest radiograph. Chest pain with pleuritic features is the second most common symptom of PE, although about one half of all patients diagnosed with PE in the ED have no complaint of chest pain.7 The classic PE pain is in the thorax between the clavicles and the costal margin that increases with cough or breathing; it is not purely substernal and not manifested from the skin or muscle. Pulmonary infarction can inflict severe focal pain, although most patients with PE and pleuritic chest pain have no radiographic evidence of pulmonary infarction. Pulmonary infarction in basilar lung segments can manifest as referred pain to either shoulder or mimic biliary or ureteral colic. Proximal PE without infarction can also cause pleuritic chest pain without focal pain.
In addition to the common symptoms of chest pain and dyspnea, approximately 3% to 4% of ED patients with PE have syncope, and another 1% to 2% present with new-onset seizure (or convulsion-like activity) or confusion.3 Because about 20% of people have a patent foramen ovale, PE that increases right-sided pressures can lead to right-to-left transit of thrombotic material in the atria and showers into the brain circulation, producing stroke-like symptoms called the paradoxical embolism syndrome. Neurologic symptoms can vary widely, from classic localized findings to staring spells, transient altered mental status, and atypical myelopathy symptoms (e.g., numbness below the waist), all of which can fluctuate.21 Presence of a patent foramen ovale worsens the prognosis in PE.
On physical examination, abnormal vital signs suggest acute cardiopulmonary stress in a patient with PE: tachycardia, tachypnea, a low pulse oximetry reading, and sometimes mild fever. Unfortunately, PE does not predictably alter any vital sign; approximately one half of patients with proven PE have a heart rate of <100 beats/min at diagnosis, and approximately one third have abnormal early vital signs that normalize in the ED.22 The mechanism of altered vital signs results in obstruction to blood flow and clot-derived autacoids, which together stimulate adrenergic efferent fibers to the heart and cause ventilation–perfusion mismatch on the lungs. The amount of clot burden does not predict vital sign changes reliably, with no clear correlation between measured clot and initial heart rate or oximetry. Although approximately 10% of patients with PE have an oral temperature of >38°C (100.4°F), <2% of patients with PE have a temperature of >39.2°C (102.5°F).
Most patients with PE have clear lungs on auscultation. Wheezes or bilateral rales make an alternative diagnosis of bronchospasm or pneumonia possible but do not exclude PE. For example, pulmonary infarction may produce rales over the affected lung segment. On heart examination, one may hear a right ventricular S3 or a split S2 with a loud second sound. The presence of a percutaneous indwelling catheters in the arm increases the probability of axillary vein thrombosis, although it is less clear whether these lines, dialysis catheters, or pacemaker wires also increase the risk of symptomatic PE.
CLINICAL FEATURES OF DEEP VEIN THROMBOSIS
Patients with DVT complain of extremity pain, swelling, or cramping. A difference of ≥2 cm between right and left leg diameter at 10 cm below the tibial tubercle doubles the likelihood of DVT. Patients presenting with upper extremity catheter-related DVT often complain of hand swelling or tightness around finger rings. About one quarter of patients with DVT have tenderness and redness in the swollen extremity, findings that are similar to those of cellulitis. Calf or saphenous vein clots are more likely to cause thrombophlebitis, defined formally as inflammation (pain, tenderness, redness, and swelling) over a vein secondary to the presence of thrombotic material in the vein. Signs and symptoms of thrombophlebitis can persist after the vein has recannulated and the clot has dissolved entirely. Calf vein thrombosis may cause Homan’s sign, which is calf pain elicited by passive foot dorsiflexion; this test has such low sensitivity and specificity that it has no predictive value.
Proximal DVT that causes complete venous obstruction leads to increased compartmental pressures, manifested as an extremely painful, swollen extremity. A swollen, painful, and pale or white limb with a proximal venous thrombosis is termed phlegmasia alba dolens, whereas a limb with a dusky or blue color is called phlegmasia cerulea dolens. Either condition poses the threat of limb loss, demanding aggressive treatment that can include thrombolysis or catheter-directed thrombectomy.
DIAGNOSIS
Routine cardiopulmonary testing in the ED generally demonstrates nonspecific findings in patients with PE. The mean pulse oximetry reading is lower in patients with proven PE than in those without PE (93 ± 2% vs 95 ± 3%), although this one signal may be absent in those with PE. Similarly, the mean partial pressure of oxygen in arterial blood (Pao2) is lower (73 ± 19 mm Hg vs 80 ± 21 mm Hg), and the difference between the estimated partial pressure of oxygen in the alveoli (Pao2) and the measured Pao2, the alveolar-arterial gradient [P(a-a)o2], is increased. The arterial partial pressure of carbon dioxide (Paco2) is usually low, reflecting a 20% to 50% increase in minute ventilation to compensate for loss of lung efficiency secondary to the increased dead space. Spontaneously breathing patients with PE also demonstrate a lower end-tidal carbon dioxide compared with healthy individuals.23
Most patients with PE have a chest radiograph with one or more abnormalities, including cardiomegaly, basilar atelectasis, infiltrate, or pleural effusion; all are nonspecific for PE. In <5% of patients, a wedge-shaped area of lung oligemia (Westermark sign—usually from complete lobar artery obstruction) or peripheral dome-shaped dense opacification (Hampton hump—always indicative of pulmonary infarction) exists. The presence of hypoxemia or dyspnea with clear lungs on physical exam and imaging suggests PE.
The 12-lead ECG is usually nonspecific, with sinus tachycardia or nonspecific ST- and T-wave changes. When PE causes the right ventricular systolic pressure to exceed 40 mm Hg, the ECG can be more specific, including T-wave inversion in leads V1 to V4, incomplete or complete right bundle-branch block, and the classic but uncommon S1-Q3-T3 pattern (Figure 56-2).24 A clinical ECG score allows severity assessment in those with diagnosed PE (higher score equates to higher mortality, Table 56-3).25
| Characteristics | Score |
|---|---|
| Tachycardia (>100 beats/min) | 2 |
| Incomplete right bundle-branch block | 2 |
| Complete right bundle-branch block | 3 |
| T-wave inversion in leads V1 through V4 | 4 |
| T-wave inversion in lead V1* | |
| <1 mm | 0 |
| 1–2 mm | 1 |
| >2 mm | 2 |
| T-wave inversion in lead V2* | |
| <1 mm | 1 |
| 1–2 mm | 2 |
| >2 mm | 3 |
| T-wave inversion in lead V3* | |
| <1 mm | 1 |
| 1–2 mm | 2 |
| >2 mm | 3 |
| S wave in lead 1 | 0 |
| Q wave in lead 3 | 1 |
| Inverted T wave in lead 3 | 1 |
| If all S1-Q3-T3 pattern, add | 2 |
| Total score (maximum: 21) |
DIAGNOSTIC TESTING FOR VENOUS THROMBOEMBOLISM
Estimating the pretest probability for VTE in a patient is the first step in selecting a diagnostic pathway. Figure 56-3 shows one diagnostic algorithm; no singular diagnostic test or algorithm perfectly excludes or diagnoses VTE. Aggressive diagnostic searches can cause harm disproportionate to benefit from hemorrhage associated with anticoagulation for a false-positive result or self-limited small clot diagnosis or from contrast nephropathy. One approach is to test further only in those with pretest probabilities of >2.5%26; those with a pretest probability of PE <2.5% are more likely to be harmed than helped by a diagnostic test, even a d-dimer assay.
FIGURE 56-3.
Pulmonary embolism rule-out criteria (PERC) rule—diagnostic algorithm for pulmonary embolism (PE). *Some physicians prefer to start with a clinical decision rule such as the Wells’ score (where <2, 2–6, and >6 are used instead of <15%, 15%–40%, and >40%, respectively). Note: Determine renal function by clinical picture (healthy, no risk factors for reduced glomerular filtration rate [GFR]) or calculated GFR. Nondiagnostic ventilation–perfusion (V̇/Q̇) scan findings require confirmation from results of another test, such as CT pulmonary angiography (CTPA), if benefits outweigh risks. + = positive for PE; – = negative for PE; Cr = creatinine; High = high probability scan findings; LMWH = low-molecular-weight heparin; Nl = normal; Nondx = nondiagnostic (any reading other than normal or high probability); quant = quantitative.
The PE rule-out criteria (Table 56-4) reliably forecast a probability of PE that is below the 2.0% test threshold in patients with a gestalt low clinical suspicion.27,28 The American College of Emergency Physicians’ Clinical Guidelines committee provided a 2b recommendation for the PE rule-out criteria rule.29 The PE rule-out criteria rule had an apparently high failure rate in one secondary analysis done in a European population with a prevalence of disease of 27% in conjunction with a low-risk Geneva score.30 However, the subsequent published erratum to the work showed that the PE rule-out criteria rule had 100% sensitivity in the same population when combined with low gestalt pretest probability as designed, which was also the case in other studies.28,31 Thus, taken together, the weight of the available information indicates that PE can be reliably excluded by the combination of a low gestalt pretest probability plus a negative PE rule-out criteria rule. However, not all patients who have any positive PE rule-out criteria must undergo an objective test for PE, since anyone over 50 years old would be tested with any finding even if suspicion was low. The key is generating a clinical gestalt first and, if low, using the PE rule-out criteria to guide further testing.
Clinical low probability (<15% probability of pulmonary embolism based on gestalt assessment) Age <50 years Pulse <100 beats/min during entire stay in ED Pulse oximetry >94% at near sea level (>92% at altitudes near 5000 feet above sea level) No hemoptysis No prior venous thromboembolism history No surgery or trauma requiring endotracheal or epidural anesthesia within the last 4 weeks No estrogen use No unilateral leg swelling, defined as asymmetrical calves on visual inspection with patient’s heels raised off the bed |
Most validated PE prediction systems categorize the patient into one of two (low or above low probability) or three (low, moderate, or higher probability) categories.33 One method uses a computerized database-derived method based on the method of attribute matching to estimate a discrete numerical percentage probability of PE.26 The Wells’ score is the most robust scoring system for categorizing the pretest probability for both PE (Table 56-5) and DVT (Table 56-6).
| Clinical Feature | Points* |
|---|---|
| Active cancer (treatment within 6 mo, or palliation) | 1 |
| Paralysis, paresis, or immobilization of lower extremity | 1 |
| Bedridden for >3 d because of surgery (within 12 wk) | 1 |
| Localized tenderness along distribution of deep veins | 1 |
| Entire leg swollen | 1 |
| Unilateral calf swelling of >3 cm (below tibial tuberosity) | 1 |
| Unilateral pitting edema | 1 |
| Collateral superficial veins | 1 |