(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
Thromboembolic diseaseDeep vein thrombosis (DVT)Pulmonary embolism (PE)HeparinCoumarinNovel oral anticoagulants (NOAC)Inferior vena caval filter (IVC Filter)DVT prophylaxisLow molecular weight heparin (LMWH)EnoxaparinFondaparinuxThrombolytic therapyPregnancyThrombophiliaSuperficial phlebitisUpper extremity DVT (UEDVT)CT pulmonary angiography (CTPA)Ventilation/perfusion scanning (V/Q)Compression ultrasound (CUS)D-dimerDeep venous thrombosis (DVT) and pulmonary emboli (PE) are usually considered the same disease, namely, thromboembolic disease (TED), as a large proportion of patients with DVT have “asymptomatic” PE and about 40 % of patients with PE have “asymptomatic DVT”. Furthermore, in most instances the treatment is the same. TED is a common disorder that carries a high mortality rate. In a population based study performed in Canada, Tagalakis and colleagues reported an incidence of venous thromboembolism of 0.90 per 1,000 person-years; the 30-day and 1-year case-fatality rates were 10.6 % and 23.0 % respectively [1]. The 1-year survival rate was 47 % in patients with cancer, 93 % in patients with unprovoked venous thromboembolism and 84 % in patients with venous thromboembolism secondary to a major risk factor. In this study 62 % of cases were associated with a major risk factor, with cancer, hospitalization, and surgery being the most common. In the International Cooperative Pulmonary Embolism Registry (ICOPER), all-cause mortality rate at 3 month was 17 % [2]. PE was considered to be the cause of death in 45 % of patients. Important prognostic factors associated with death from pulmonary embolism were age older than 70 years, cancer, congestive heart failure, chronic obstructive pulmonary disease, systolic arterial hypotension, tachypnea, and right ventricular hypokinesis on echocardiography.
Pregnancy, Venous Thromboembolism and Thrombophilias
TED is one of the leading causes of maternal morbidity and mortality. The incidence of VTE is estimated at 0.76–1.72 per 1,000 pregnancies [3, 4] In the United Kingdom, VTE accounts for a third of all maternal deaths [5, 6]. In women of reproductive age, over half of all TED thrombotic events are related to pregnancy [7]. The risk of venous thrombotic events is increased fivefold during pregnancy and 60-fold in the first 3 months after delivery compared with non-pregnant women [7]. Similarly the risk of PE is increased two fold during pregnancy and up to 30-fold in the post-partum period [7]. DVTs occur with equal frequency during each trimester. Approximately, one third of pregnancy related DVT and half of pregnancy related PE occur after delivery. Regardless of the type of event, the postpartum period carries the highest daily risk of VTE. Most cases of postpartum DVT occur within the first 4 weeks with the highest number of cases occurring in the second week [7]. However, the risk remains increased for up to 3 months postpartum.
Up to 50 % of cases of VTE in pregnancy are associated with an inherited or acquired thrombophilia [8, 9]. A thrombophilia is defined as a disorder of hemostasis that predisposes an individual to a thrombotic event [10]. The relative prevalence of the inherited thrombophilias is variable and depends upon the population studied (see Table 27.1) [10–12]. Although in combination the described inherited thrombophilias are common (affecting 15 % of Western populations) and underlie approximately 50 % of VTE in pregnancy, VTE complicates only 0.1 % of pregnancies. Therefore, the presence of a thrombophilia alone, even in the context of the hypercoagulable state of pregnancy, does not consistently result in a thrombotic event. This suggests that multiple interacting prothrombic factors are responsible for VTE in pregnant patients.
Thrombophilic defect | Prevalence (%) | OR (95 % CI) |
|---|---|---|
Factor V Leiden heterozygous | 2–7 | 8.3 (5.4–12.7) |
Factor V Leiden homozygous | 0.5–0.25 | 34.4 (9.9–120.1) |
Prothrombin G20210A heterozygous | 2 | 6.8 (2.5–18.8) |
Prothrombin G20210A homozygous | Rare | 26.4 (1.24–559.3) |
Antithrombin deficiency (<80 % activity) | 0.02–0.55 | 4.7 (1.3–16.9) |
Protein C deficiency (<75 % activity) | 0.2–0.33 | 4.8 (2.2–10.6) |
Protein S deficiency (<65 % activity) | 0.03–0.13 | 3.2 (1.5–6.9) |
Methyltetrahydrofolate reductase mutation (homozygous) | – | 0.74 (0.22–2.48) |
Antiphospholipid antibodies | 1–8 | 15.8 (10.9–22.8) |
Site of Venous Thrombosis
Venous thrombosis may involve the lower limb, upper limb, iliac vein and in rare circumstances other veins. Thromboses of the limbs may be further classified as proximal or distal and superficial or deep [13]. The location of the venous thrombosis is critically important, particularly in the lower limb, as it determines the therapeutic approach. It is believed that most lower extremity deep venous thromboses originate in the distal lower limb leg with about a third of these clots extending above the knee into the proximal veins. The risk of PE is high with proximal lower extremity DVT, whereas the risk of PE is believed to be exceedingly low with isolated lower extremity DVT. It is therefore important to distinguish between a proximal and distal lower extremity DVT (see below). The risk of PE appears to be lower with upper extremity DVT and superficial venous thrombosis of the extremities. The incidence of isolated DVT in the iliac vein is thought to be relatively higher in pregnant women [14, 15].
The Veins of the Lower Limb
In managing patients with lower limb venous thrombosis it is of utmost importance to have a good understanding of the venous system of the lower leg (see Fig. 27.1). A source of ongoing confusion and a “potentially lethal misnomer” is the term “superficial femoral vein” which describes a vein that is not superficial [16]. The femoral vein is contained within the deep muscle compartments bound by the muscle fascia and is a “deep” vein. DVTs above the knee (but including involvement of the popliteal vein) are considered proximal deep venous thromboses of the lower extremity.
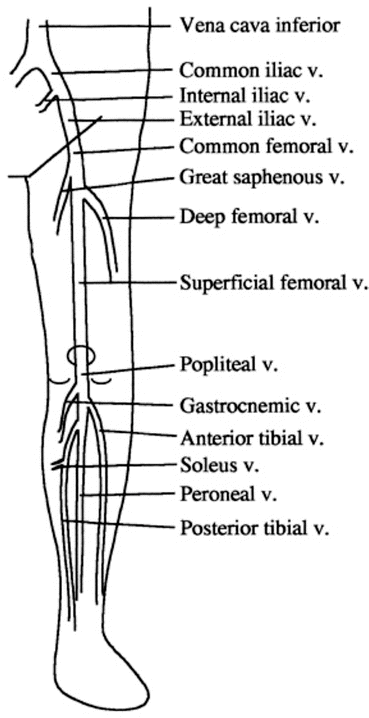

Fig. 27.1
Deep veins of the lower limb
Critically ill ICU patients have many factors which increase the risk of DVT. The risk of DVT can be quantified according to the modified Caprini score (see Table 27.2) [17–19]. Using this risk assessment tool most ICU patients fall into the high risk or very high risk group. Consequently ALL ICU patients require DVT prophylaxis (mechanical, pharmacologic, or both) on admission to the ICU. Despite universal guidelines recommending DVT prophylaxis in all ICU patients, in the XPRESS trial only 50 % of the patients were receiving DVT prophylaxis [20]. Ho et al. demonstrated that omission of thromboprophylaxis within the first 24 h of ICU admission was associated with an increased risk of mortality (OR, 1.22; CI 1.15–1.30, p = 0.001) [21].
Table 27.2
Modified Caprini DVT risk assessment score
1 point | 2 points | 3 points | 5 points |
|---|---|---|---|
Age 41–60 | Age 61–74 | Age over 75 years | Elective arthroplasty |
Minor surgery | Major surgery or laparoscopic surgery (>45 min) | History of VTE | Hip, pelvis or leg fracture |
Varicose veins or swollen legs | Arthroscopic surgery | Any thrombophilia | Acute spinal cord injury |
Inflammatory bowel disease | Malignancy | Stroke | |
Obesity (BMI > 25) | Confined to bed >72 h | Multiple trauma | |
Congestive heart failure | Immobilizing plaster cast | ||
Sepsis | Central venous access | ||
Chronic lung disease | |||
Medical patient at bed rest | |||
Pregnancy or postpartum | |||
Oral contraceptive or hormone replacement |
While few head-to-head randomized studies have been performed, sequential compression devices (SCDs), unfractionated heparin (UFH) and low molecular weight heparin (LMWH) appear to be equally effective in reducing the risk of DVT in low to moderate risk patients [22–25]. In high risk patients (orthopedic patients) SCDs appear to be as effective as LMWH in preventing DVTs [26–28]. In high risk patients LMWH and fondaparinux appear to be more effective in preventing asymptomatic DVT than UFH [29–31]. The combination of SCDs and LMWH appear to act additively in reducing the risk of DVT [31–33]. Graduated compression stockings, however, appear to have no role in preventing DVT [32]. These data suggest that in high to very high risk patients a combination of pharmacologic prophylaxis (if not contraindicated) together with SCD’s are indicated [19]. As is evidenced from Table 27.2 trauma patients are at a very high risk of DVT. LMWH has been demonstrated to be safe and effective in trauma patients and is the approach recommended by the East Surgical Group and the current ACCP guidelines [34, 35]. In trauma patients at high risk of bleeding the placement of a removal IVC filter may be a suitable strategy [36].
In a meta-analysis comparing any heparin formulation to placebo the risk of DVT and PE was 50 % lower with heparin [37]. Trials testing UFH used only bid dosing. Although there are no direct comparisons of bid versus tid UFH in any population, indirect comparisons suggest that their effects are similar on thrombosis and bleeding. The Canadian Critical Care Trial group performed a multi-center trial, in which they compared dalteparin (a LMWH) to unfractionated heparin (at a dose of 5,000 IU twice daily) in 3,764 patients [22]. There was no significant difference between the rates of proximal DVT, which occurred 5.1 % of patients receiving dalteparin versus 5.8 % receiving UH. The proportion of patients with PE was significantly lower with dalteparin (1.3 %) than with UH (2.3 %); the explanation for this finding and its implications are unclear. There was no significant difference in the rates of major bleeding or death in the hospital.
The use of sequential compression devices (SCDs) and graduated compression stockings (GCS) in the prevention of DVT has been controversial. However, as discussed above SCDs appear to be as effective as UF and LMWH in reducing the risk of DVT. In an observational propensity adjusted study Arabi et al. demonstrated that the use of SCDs was associated with a significantly lower VTE incidence compared with no mechanical thromboprophylaxis (RR 0.45; 0.22–0.95; p = 0.04) [38]. In this study GCS were not associated with a lower risk of VTE. Vignon randomized 407 patients with a high risk of bleeding to receive SCDs and GCS or GCS alone for 6 days during their ICU stay [39]. By day 6, the incidence of TED was 5.6 % in the SCD + GCS group and 9.2 % in the GCS group (RR 0.60; CI 0.28–1.28; p = 0.19). Lacut et al. randomized 151 patients who had suffered an intracerebral bleed to SCD + GCS or GCS alone [40]. Asymptomatic DVTs were detected in 4.7 % in the SCD + GCS group and 15.9 % in the GCS group (RR 0.29; CI 0.08–1.00). The CLOTS 3 trial randomized 2,876 immobile patients who had suffered a stroke to SCDs or no SCDs [41]. The primary outcome (DVT in the proximal veins detected on a screening CDU or symptomatic DVT in the proximal veins within 30 days of randomization) occurred in 8.5 % of patients allocated SCD and 12.1 % of patients allocated no SCD; an absolute reduction in risk of 3.6 % (95 % CI 1.4–5.8). The CLOTS 1 study randomized 2,518 patients who had suffered a stroke to thigh-length GCS or no GCS [42]. The primary outcome occurred in 10.0 % patients allocated to thigh-length GCS and in 10.5 % who did not receive GCS. These studies provide robust data that SCD reduce the risk of DVT while GCS do not.
The current American College of Chest Physicians (ACCP) guidelines suggest “For acutely ill hospitalized medical patients at increased risk of thrombosis, we recommend anticoagulant thromboprophylaxis with LMWH, UH bid, UH tid, or fondaparinux (Grade 1B)” [43]. Furthermore, they recommend “For acutely ill hospitalized medical patients at increased risk of thrombosis who are bleeding or at high risk for major bleeding, we suggest the optimal use of mechanical thromboprophylaxis with SCD. When bleeding risk decreases, and if VTE risk persists, we suggest that pharmacologic thromboprophylaxis be substituted for mechanical thromboprophylaxis” (Grade2B). The reader is referred to the “American College of Chest Physicians evidence-based clinical practice guidelines on the Prevention of venous thromboembolism (9th Edition)” for a comprehensive review on this topic [43]. Decousus et al. evaluated the independent risk factors for bleeding in a large cohort of medical patients [44]. These authors then developed a bleeding score risk to quantitate the risk of bleeding (see Table 27.3). Patients with a bleeding risk score ≥7 were at an increased risk of bleeding. While this scoring system has not been independently validated it provides a useful tool to weigh the risks and benefits of pharmacological DVT prophylaxis in medical patients [45].
Table 27.3
Bleeding risk score
Bleeding risk factor | Points |
|---|---|
Moderate renal failure, GFR 30–59 | 1 |
Male vs. female | 1 |
Age > 40–84 | 1.5 |
Current cancer | 2 |
Rheumatic disease | 2 |
Central venous catheter | 2 |
ICU/CCU | 2.5 |
Severe renal failure, GFR < 30 | 2.5 |
Hepatic failure (INR > 1.5) | 2.5 |
Age ≥ 85 | 3.5 |
Platelet count <50 | 4 |
Bleeding in the 3 months before admission | 4 |
Active peptic ulcer | 4.5 |
It is important to note that LMWHs and fondaparinux are renally excreted (and therefore should not be used when GFR < 35 mL/min), will accumulate in renal failure, and that the anticoagulant activity of these drugs are not easily reversed. Prophylactic vena-caval filters have very limited utility for DVT prophylaxis; their use is associated with significant long term sequela (recurrent DVT and post-phlebitic syndrome). Vena-caval filters are frequently placed in trauma patients, because of the perceived risk of pharmacologic prophylaxis. However, a large randomized clinical trial supports the fact that pharmacologic prophylaxis is both safe and effective in these patients [34]. Furthermore, there are no randomized prospective evaluations of the use of vena-caval filters in this setting.
Despite adequate prophylaxis, DVTs develop in between 5 and 10 % of ICU patients [20, 22, 46]. Furthermore, these DVTs’ are frequently “clinically silent”. These observations have led to the idea of routinely screening ICU patients for DVT with compression ultrasound. However, an economic and decision analysis by Sud and colleagues concluded that “appropriate prophylaxis provides better value in terms of costs and health gains than routine screening for deep vein thromboses” [47]. In this study weekly screening Doppler compression ultrasound cost more than $200,000 per QALY gained.
Suggested DVT Prophylaxis Protocols
Enoxaparin (or equivalent LMWH) 40 mg SC once daily OR fondaparinux 2.5 mg SC once daily OR UH 5,000 U BID. UH should be avoided in patients at high risk of HIT. See dosing adjustments in Tables 27.4 and 27.5.
Table 27.4
Fondaparinux dosing
Fondaparinux dosing (kg)
Prophylaxis
Treatment (mg)
<50
Heparin 500 U SC BID
5
50–100
2.5 mg daily
7.5
>100
2.5 mg daily
10
Table 27.5
Enoxaparin dosing with renal dysfunction and obesity
Indication
Creatinine Cl (mL/min)
Standard dose
Prophylaxis Medical
>30
40 mg SC daily
0.5 mg/kg SC daily
<30
30 mg SC daily
Heparin 5,000 U SC BID + SCDs
<10
Heparin 5.000 U SC BID
As above
Prophylaxis Surgery
>30
30 mg SC q 12
40 mg SC q 12 + SCDs
<30
30 mg SC daily
Heparin 5000 U SC BID + SCDs
<10
Heparin 5,000 U SC q 12
As above
VTE treatment
>30
1 mg/kg SC q 12 or 1.5 mg/kg SC daily
Body Weight <190 kg
1 mg/kg SC q 12
Body Weight > 190 kg
1 mg/kg SC q 12+
Monitor anti-Xa
Or heparin infusion
<30
1 mg/kg SC daily
Heparin infusion
<10
Heparin infusion
Heparin infusion
Very high risk for DVT (cancer, paralytics, previous DVT, morbid obesity, etc): Enoxaparin 40 mg SC once daily OR fondaparinux 2.5 mg SC once daily PLUS SCD’s
Reduce dose of Enoxaparin to 30 mg SC once daily with renal impairment
In patients with renal failure (GFR < 35 mL/min) UFH 5,000 U BID PLUS SCD’s
Neurosurgical patients/Intracerebral hemorrhage: SCD’s. Add UFH 5,000 U BID if no hematoma expansion at 48 h.
Diagnosis of DVT
Compression ultrasound (CUS) is a non-invasive test with a sensitivity of 97 % and a specificity of 94 % for the diagnosis of symptomatic, proximal lower extremity DVT in the general population [48]. Compression ultrasound is less accurate for isolated calf and iliac vein thrombosis [49]. Magnetic resonance direct thrombus imaging (MRDTI) which has no radiation exposure and is not deleterious to the fetus, has a high sensitivity and specificity for the diagnosis of iliac vein thrombosis [15, 50]. Pulsed Doppler of the iliac vein and CT scanning may be useful for detecting iliac vein thrombosis when MRI is not available [51, 52].
Distal Lower Extremity DVT
The ACCP guidelines suggest; “In patients with acute isolated distal DVT of the leg and without severe symptoms or risk factors for extension, we suggest serial imaging of the deep veins for 2 weeks over initial anticoagulation” [53]. Furthermore, “In patients with acute isolated distal DVT of the leg and severe symptoms or risk factors for extension, we suggest initial anticoagulation” [53]. Anticoagulation is preferable in the immobilized ICU patient, particularly if there is extensive clot burden.
Upper Extremity DVT
Upper extremity DVT (UEDVT) is not uncommon in the ICU. Risk factors include central venous catheters, malignancy, previous lower extremity DVT and inherited disorders of coagulation [54–56]. Malinoski et al. screened 862 surgical and trauma ICU patients for an UEDVT using duplex ultrasound [57]. In this study 15 % of patients had an UEDVT. The internal jugular vein was the most common site (52 %), 72 % were non-occlusive and 64 % were associated with a central venous catheter. The CVC was removed in 73 of the 79 catheter associated UEDVT. Line removal was associated with a significantly greater occurrence of clot improvement on the follow up duplex. Lamontague and colleagues screened a cohort of 3,746 medical-surgical ICU patients who were receiving pharmacologic thromboprophylaxis for the presence of non-leg DVT (NLDVT) [13]. In this study 2.2 % of patients developed one or more NLDVT. Cancer was the only independent predictor of NLDVT. NLDVT increased the risk of PE (HR 11.83) but did not increase the risk of death. In this study 94.5 % of NLDVT’s occurred in the upper extremity.
As is the case with lower extremity DVT most cases of upper extremity DVT are asymptomatic. Presenting features include pain and swelling of the extremity. These features should prompt a Doppler ultrasound examination. Complications of upper extremity DVT include PE and the postthrombotic syndrome [55]. While there are no RCT’s to guide the management of this condition, the recommended treatment is anticoagulation with UFH or LWMH followed by 3 months of Coumadin [58]. In patients with a catheter associated UEDVT it is advisable to start anticoagulation before removal of the catheter to limit the risk of clot embolization.
Superficial Phlebitis
Superficial phlebitis refers to the clinical findings of pain, tenderness, induration and/or erythema in one of the superficial veins due to inflammation, infection and/or thrombosis. The term “superficial venous thrombosis” is preferred when the presence of clot is confirmed (by ultrasonography) and the term “superficial phlebitis” in the absence of venous thrombosis. Patients with inherited thrombophilic states have an increased risk of superficial venous thrombosis [59, 60]. In addition, obesity and immobilization increase the risk of superficial venous thrombosis. The differential diagnosis includes DVT, cellulitis, lymphangitis, insect bite and erythema nodosum. Superficial and deep venous thrombosis can occur together because of direct extension of the superficial venous clot into the deep venous system; therefore, all patients with suspected superficial venous thrombosis should undergo CUS examination [61]. Superficial venous thrombosis of the great saphenous vein, particularly when the clot extends to the sapheno-femoral junction, in is associated with an increased risk of DVT [61, 62]. In a large prospective, epidemiologic study, Decousus and coauthors reported that among 844 patients with superficial venous thrombosis 25 % had concomitant DVT or PE [63]. Furthermore, among the 600 patients without DVT or PE at enrollment, 10 % developed thromboembolic complications at 3 months despite the fact that 90 % received anticoagulants.
A systemic review which included 24 studies involving almost 2,500 cases concluded that treatment with LMWH should be considered to prevent thromboembolic events and the extension and/or recurrence of superficial venous thrombosis [64]. This review suggested treatment with an “intermediate dose of LMWH for at least a month.” Furthermore, the review concluded that “the optimal dose and duration of anticoagulation requires further investigation and the role of NSAIDS alone or in combination with LMWH remains uncertain” [64, 65]. In patients with thrombosis of the great saphenous vein it may be prudent to repeat CUS before stopping treatment with LMWH.
Pulmonary Embolism
Patients may be admitted to the ICU with hypoxemic respiratory failure with the diagnosis of PE or in whom a PE is suspected. The diagnosis of PE should always be entertained in patients who present to the ICU with hypoxemic respiratory failure in whom the diagnosis is uncertain. In addition, PE may be the cause of a “COPD exacerbation” or worsening heart failure [66]. PE may develop in ICU patients admitted to the ICU for other reasons. In the ICU setting, PE may cause acute episodes of hemodynamic instability or hypoxia and may contribute to failure of weaning from mechanical ventilation. However, in many instances PE is “clinically silent” and unsuspected. Consequently, PE is one of the most common unexpected autopsy findings in the critically ill, being reported in between 7 and 27 % of autopsies [67, 68]. PE is considered massive when it causes hemodynamic compromise (hypotension) and sub-massive when it is associated with tachycardiac and RV dysfunction.
Diagnosis of Pulmonary Embolism
The diagnosis of PE is one of the more challenging dilemmas in clinical medicine. Scoring systems have been used to stratify patients as having a high or low risk venous thromboembolism. For suspected pulmonary embolism, two scores are widely used: the Wells score [69] and the revised Geneva score [70]. The Wells score can be used to diagnose suspected deep vein thrombosis [71]. These scoring system are presented in Tables 27.6, 27.7, and 27.8. These rules have similar predictive accuracy [72]. While an arterial blood gas analysis, electrocardiogram (ECG) and chest radiograph (CXR) should be performed in all patients suspected of having a PE, these tests have a low specificity for the diagnosis of PE. Fibrin D-dimer is a degradation product of cross-linked fibrin, and its concentration increases in patients with acute venous thromboembolism. When assayed by a quantitative ELISA or by automated turbidimetric assays, D-dimer is highly sensitive (more than 95 %) in excluding acute DVT or PE, usually below a threshold of 500 μg/L; i.e. D-dimer has a very high negative predictive value [73]. In elderly patients (50 years and older) a threshold of age x 10 allows for a larger number of patients to be ruled out for PE with a low likelihood of false negative tests [74]. Compression ultrasonography (CUS) has largely replaced venography as the main imaging procedure to diagnose DVT. Ultrasound has a lower sensitivity and specificity for the detection of calf vein thrombosis than it does for proximal DVT.
Table 27.6
Wells score for DVT
Wells Score for DVT | Points |
|---|---|
Cancer | +1 |
Paralysis or recent plaster cast | +1 |
Bed rest >3 days or surgery <4 weeks | +1 |
Pain on palpation of deep veins | +1 |
Swelling of entire leg | +1 |
Diameter difference on affected calf > 3 cm | +1 |
Pitting edema (affected side) | +1 |
Dilated superficial veins (affected side) | +1 |
Alternative diagnosis at least as probable as DVT | −2 |
Table 27.7
Wells score for PE
Wells Score for PE | Points |
|---|---|
Previous PE or DVT | +1.5 |
Heart rate > 100/min | +1.5 |
Recent surgery or immobilization | +1.5 |
Clinical signs of DVT | +3 |
Alternative diagnosis less likely than PE | +3 |
Hemoptysis | +1 |
Cancer | +1 |
Table 27.8
Revised Geneva Score for PE
Revised Geneva Score for PE | Points |
|---|---|
Age > 65 | +1 |
Previous DVT or PE | +3 |
Surgery or lower limb fracture within 1 month | +2 |
Active malignancy | +2 |
Unilateral leg pain | +3 |
Hemoptysis | +2 |
Heart rate 75–94/min | +3 |
Heart rate > 95 beats/min | +5 |
Pain on deep vein palpation in leg and unilateral edema | +4 |
Pulmonary angiography has traditionally been considered the gold standard with which to compare other methods, however, angiography is invasive, costly and not readily available in most hospitals. Ventilation/perfusion scanning (V/Q) currently has a limited role in the diagnosis of PE. While a completely normal V/Q scan rules out a PE and a high probability scan effectively rules in PE, most V/Q scans are of indeterminate probability and neither rule in or rule out a PE. CT pulmonary angiography (CTPA) has largely replaced V/Q lung scintigraphy as the main imaging modality in suspected PE. CTPA has the additional advantage of allowing visualization of the lung parenchyma allowing the formulation of alternative diagnoses. Single detector CTPA has a sensitivity of only about 70 %. Multidetector CTPA is more sensitive than single-detector CT angiography. In the PIOPED II study (conducted between 2001 and 2003) multidetector CTPA had a sensitivity 83 % and a specificity 96 %; this increased to 90 % and 95 % respectively with CT venography (of the lower limbs) [75]. Consequently, CUS of the lower extremities was recommended in patients with a negative CTPA to improve the diagnostic sensitivity. However, more recently the added benefit of CUS with a negative CTPA has been questioned [73]. Righini et al. compared two diagnostic strategies: clinical probability assessment and either D-dimer measurement and CTPA (DD-CT strategy) or D-dimer measurement, CUS and CTPA [76]. In the DD-US-CT strategy, D-dimer was measured only in patients with a low or intermediate clinical probability on the revised Geneva score. In these patients, pulmonary embolism was ruled out by a negative D-dimer test without further testing. When the D-dimer concentration was greater than 500 ng/mL, CUS was performed in both legs, and patients with a proximal DVT were given anticoagulant drugs without further testing. Patients without proximal DVT underwent CTPA and were treated if positive for PE. The DD-CT strategy was similar except that a CUS was not performed. The primary outcome was the 3-month thromboembolic risk in patients who were left untreated on the basis of the exclusion of PE by diagnostic strategy; the primary outcome occurred in 0.3 % in the DD-US-CT group and 0.3 % in the DD-CT group. These data suggest that CUS may not be necessary in all patients with a suspected PE and a negative CTPA. However, it would appear prudent to perform a CUS in those patients who are admitted to hospital and require further diagnostic workup. Isolated subsegmental abnormalities, which are reported in 10–20 % of CTPA’s may be due to PE causing symptoms or incidental PE that is not responsible for the patient’s symptoms or may be a false positive finding [77]. Consequently, it is uncertain if patients with these findings should be treated. Treatment is generally recommended in those patients with clinical evidence of PE (high D-dimer, etc) and a low risk of bleeding [77].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





