Patricia Polgar-Bailey Tuberculosis (TB) is an airborne infectious disease caused by Mycobacterium tuberculosis, an acid-fast aerobic bacterium that is capable of remaining alive outside the host for a relatively long time. In the United States, most cases of TB are caused by M. tuberculosis, also referred to as the tubercle bacillus. However, several closely related Mycobacterium species can cause disease in humans, including Mycobacterium bovis, the cause of TB in cattle; Mycobacterium avium, one of the causes of TB in birds; and Mycobacterium africanum. TB caused by these organisms was relatively rare in the United States until they were identified as the cause of opportunistic infections in patients infected with human immunodeficiency virus (HIV). TB has been described as the greatest killer in history, claiming more lives than any other infectious disease.1 Globally, TB transmission continues despite highly effective frontline combination therapy, a widely administered vaccine, intensive control efforts, and the allocation of tremendous resources to improve interventions.2,3 There are an estimated 8.6 million new case of TB and 1.3 million deaths from the disease annually.4 In 2014, globally approximately 480,000 people developed multidrug-resistant tuberculosis (MDR-TB) and more than half of these cases were in India, China, and the Russian Federation.5 The increasing incidence and transmission of TB is largely a result of the spread of HIV in Africa and to the economic difficulties and associated decline in health infrastructure in eastern Europe and central Asia as well as increased reporting. Approximately 80% of all TB cases are found in 22 “high-burden” countries, most of which are in Africa and Asia. India has the highest rate of TB mortality, followed by China, where there are 150,000 TB-related deaths annually.4 Persons with active TB who receive no treatment can infect an average of 10 to 15 people annually.6 Southeast Asia currently has the highest prevalence of TB, with one third of new cases occurring in this area every year, but the incidence per capita is highest in sub-Saharan Africa, where the presence of TB parallels the HIV and acquired immunodeficiency syndrome (AIDS) epidemic. Two billion people have latent tuberculosis infection (LTBI), the presence of M. tuberculosis in the body without signs and symptoms or radiographic or bacteriologic evidence of TB; without treatment, approximately 5% to 10% of these individuals will progress to active disease at some point in their lifetime.1,7 In the United States, approximately 9 to 14 million people have LTBI.1 During the mid-20th century, the United States benefited from relatively successful control of TB. From 1953 to 1985, the reported cases of TB in the United States dropped from 84,000 to 22,000.8 From 1985 to 1992, there was an unprecedented resurgence of TB in the United States. Since 1993, the annual TB rate has decreased steadily. In 2013, the TB case rate in the United States was 3.0 cases per 100,000 (30 per million), decreased from 3.8 per 100,000 persons in 2009. Nine states and the District of Columbia reported case rates above the national average of 3.0 cases per 100,000.9 Hispanics (29%) represented the largest percentage of total cases. Asians (28%) constituted the second largest percentage of cases, surpassing non-Hispanic blacks and African Americans. Foreign-born persons represent a disproportionate percentage (59%) of the national case total; in 2007, the TB case rate was more than 10 times as high among foreign-born as among U.S.-born residents.10,11 Asians represent the largest percentage of foreign-born cases, and Asians born outside of the United States represent 44% of the TB cases in foreign-born persons and 26% of the national case total. Although the annual incidence of TB has decreased, the percentage of primary drug-resistant cases increased to 1.2% in 2009 compared with 1.0% in 2008. Primary drug resistance is defined as no previous history of TB and resistance to at least isoniazid (INH) and rifampin, the most potent first-line antitubercular drugs.1 MDR-TB emerged during the 1990s as a significant threat to TB control, both in the United States and worldwide, and remains a significant threat to TB control. MDR-TB treatment requires the use of second-line drugs that are less effective, more toxic, and costlier than first-line INH- and rifampin-based regimens. In addition, MDR-TB is associated with higher morbidity and mortality than non–drug-resistant TB.1 Historically, TB in the United States was a disease that affected primarily older adults; increasingly, younger adults and children are being affected, particularly foreign-born children and adolescents.7,12 An estimated 11% of TB cases worldwide occur in children younger than 15 years. In TB-endemic areas, children are at increased risk of acquiring TB because of the increased likelihood of close contact and exposure to adults with TB. Progression from infection to disease (approximately 8% to 10% overall) is higher for children of all ages and highest for infants younger than 1 year (43%) and children aged 1 to 5 years (24%). Of additional concern are those children who do not progress from infection to active disease in childhood but who constitute a potential pool for disease in adulthood.12 Many factors have contributed to the increased incidence of TB, including the HIV epidemic and higher rates of poverty, homelessness, incarceration, and drug use. An increasing number of immigrants, many of whom live in crowded housing and have inadequate health care, and an increasing number of residents in long-term care facilities have also contributed to this public health problem. Deterioration in the health care infrastructure and reductions in TB outreach programs, which historically improved compliance with treatment regimens, have also contributed to the resurgence of TB. Alcohol and illegal drug use increase the risk of TB transmission and act as barriers to TB control and prevention.13 Substance abuse decreases the likelihood of seeking medical care and adhering to and completing therapy. In addition, the use of substances often takes place in enclosed crowded spaces with poor ventilation, which increases the risk of TB exposure and transmission. In the United States, approximately one of three U.S.-born persons aged 15 years with TB also abuses substances. TB is largely a social disease, and homeless and incarcerated individuals are at increased risk of infection with TB.14,15 TB control can be particularly problematic in correctional and detention facilities, in which persons from diverse backgrounds and communities live together for varying and sometimes extended periods. In July 2006, the Centers for Disease Control and Prevention (CDC) published guidelines for the prevention and control of TB in jails, prisons, and other correctional and detention facilities.16 Providers working in these settings should familiarize themselves with the recommendations, which can be found in Morbidity and Mortality Weekly Report (MMWR) or on the Internet at www.cdc.gov/mmwr/preview/mmwrhtml/rr5509a1.htm. Transmission of M. tuberculosis in health care institutions was a contributing factor to the resurgence of TB during the period from 1985 to 1992, and recommendations were developed to prevent transmission in these settings. However, the elevated risk among health care workers may be attributable to other factors (e.g., birth in a country with a high incidence of TB). A recent large multistate occupational survey indicated that health care workers, with the exception of respiratory therapists, do not have a higher risk for TB than the general population.6 The decelerating decline of the overall national TB rate, the persistent disparities in TB rates between U.S.-born and foreign-born persons and between whites and ethnic minorities, and the increase in MDR-TB cases all threaten progress toward the goal of eliminating TB in the United States.8 Major challenges to successful control of TB in the United States include detection and treatment of TB in the non–U.S.-born population, elimination of delays in detecting and reporting cases of pulmonary TB and protecting contacts of TB-infected persons, and prevention of and response to TB outbreaks. In addition, there is a large reservoir of persons living in the United States with LTBI who are at risk for progression to TB disease. Finally, the successful control of TB depends on maintenance of clinical and public health expertise in TB management in an era of declining TB incidence.6 Treatment of TB benefits both the individual patient and the community as a whole. Therefore, any public health program or health care provider undertaking to treat a patient with TB is assuming a public health function that includes not only prescribing an appropriate medication regimen but also ensuring adherence to the regimen until treatment is completed.17 According to a joint statement by the American Thoracic Society, CDC, and Infectious Diseases Society of America, the responsibility for successful treatment of TB is assigned to the public health program or private provider rather than to the individual with TB.18 TB is spread primarily through direct infection (person to person), but it can also be spread indirectly by the airborne transmission of the tubercle bacilli, which can remain suspended in the air for several hours. Transmission, which may occur if these bacilli-laden sputum droplets (each containing one to three organisms) are inhaled, depends on three factors: the infectiousness of the person with TB, the environment in which the exposure occurred, and the duration of exposure. Although theoretically one organism implanted in the alveolus can initiate this process, 5 to 200 organisms are usually required.19 Most of the larger inhaled particles become lodged in the upper respiratory tract, where infection is unlikely to take place. Infection begins if the droplet nuclei reach the alveolar macrophage and multiplication of the tubercle bacilli is initiated. A small number of mycobacteria spread through the lymph system to regional lymph nodes and through the bloodstream to more distant tissues and organs, including areas in which TB is more likely to develop, such as the apices of the lung, the kidneys, the brain, and the bone. Eighty-five percent of all TB cases involve the lungs; other common sites include the pleura, central nervous system (CNS), lymphatic system, genitourinary system, and bones and joints. TB can also become disseminated and then is referred to as miliary TB. TB disease has two distinct epidemiologic patterns. Reactivation, or postprimary disease, is the most common clinical form of TB. Most symptomatic cases of TB arise in persons with a history of TB infection who were inadequately treated or not treated. The second epidemiologic profile, primary infection, does not usually appear as a symptomatic infection except in persons infected with HIV. More than 90% of persons with primary infection are entirely asymptomatic, and infection with TB is identified only by a positive reaction to a tuberculin skin test (TST). Certain medical conditions and other factors increase the risk that LTBI will progress to active TB disease. The risk may be three times greater (as with coexistent diabetes mellitus) to 100 times greater (as with HIV infection) for persons who have these conditions compared with those who do not.7 Medical conditions and other factors associated with progression from LTBI to TB disease are listed in Box 235-1. Persons who have been infected with M. tuberculosis but do not have active disease (LTBI) are completely asymptomatic. For the majority of persons, the only evidence of LTBI is an immune response against mycobacterial antigens, which is demonstrated by the Mantoux test or interferon gamma release assays (IGRAs). Two U.S. Food and Drug Administration (FDA)–approved IGRAs are commercially available in the United States: QuantiFERON-TB Gold In-Tube (QFT-GIT) test and T-SPOT.TB test.20 There is no radiographic evidence of TB in persons with LTBI. Symptoms of pulmonary TB (the most common site) include fatigue, anorexia, weight loss, night sweats, cough, chest pain, hemoptysis, irregular menses, and low-grade fever. Symptoms in adults are often subtle and may appear in conjunction with or simulate other illnesses and therefore are often not associated with TB. However, one third of persons with pulmonary TB are asymptomatic on initial presentation.7 Approximately 15% of cases of TB are extrapulmonary; common sites include the bones and joints, genitourinary system, lymphatic system, and CNS. The symptoms of extrapulmonary TB depend on the site affected. TB of the spine often causes back pain, whereas TB of the genitourinary system may result in hematuria or persistent dysuria. A complete physical examination is an essential part of the evaluation but cannot be used alone to confirm or to exclude TB. Even if the physical examination findings are entirely normal, it can provide useful information about the patient’s overall condition. Certain findings, although not diagnostic of TB, may be suggestive of the diagnosis. Rales in the upper posterior portion of the chest, evidence of pleural effusion, lymphadenopathy, weight loss, and fever may increase the suspicion for TB. Confirmation of TB is based on the diagnostic evaluation presented in the next section. Screening is the first step in the diagnostic evaluation of TB and is performed to identify infected patients at high risk for TB who would benefit from preventive therapy as well as patients with TB who need treatment. If a person is infected with TB, a reaction to the TST is detectable 2 to 8 weeks after infection.7 Because most patients infected with TB are asymptomatic, health care providers should administer the TST to all high-risk persons as part of their routine evaluation. Persons with any of the medical conditions or other factors listed in Box 235-1 should be screened annually unless there is prior documentation of a positive TST reaction. Other high-risk groups include close contacts of a person with infectious TB disease; foreign-born persons from areas in which TB is common (e.g., Asia, Africa, and Latin America); the medically underserved and low-income populations, including high-risk racial and ethnic groups (e.g., Asians and Pacific Islanders, African Americans, Hispanics, and Native Americans), migrant farm workers, and homeless persons; residents of long-term care facilities (e.g., correctional facilities and nursing homes); and other groups identified as having a disproportionate prevalence of TB. Routine institutional screening is also recommended for health care workers and the staff of long-term institutional facilities who may have occupational exposure to TB or who would pose a risk to large numbers of susceptible persons if they developed active disease (e.g., staff member of an AIDS hospice).7 The standard and preferred method of screening for TB infection is the Mantoux test, which is administered by injection of 5 tuberculin units (0.1 mL) of purified protein derivative (PPD) solution intradermally into either the volar or the dorsal surface of the forearm. The injection should be made with a disposable tuberculin syringe with the needle bevel pointing upward. The injection should produce a discrete, pale elevation of the skin (a wheal) that is 6 to 10 mm ( The skin test result is read within 48 to 72 hours. If the patient fails to show up for a scheduled reading within 72 hours, a positive reaction may still be measurable up to 1 week later. A TST must be repeated if the result was not measured and recorded in millimeters of induration.7 TST results should be measured only by a trained health care professional. Patients or family members should not measure TST results. The TST should be repeated for all negative responses not documented within 72 hours.1 The criteria to determine whether a skin test result is significant depends on a patient’s risk for development of disease or ability to mount a reaction to the PPD. The criteria for a positive TST reaction are listed in Box 235-2. Once a patient has had a positive TST reaction, no subsequent tuberculin skin testing should be performed. The TST should never be performed on a person who has had a previous positive TST reaction or who has had treatment of TB disease.7 A variety of factors can cause a false-negative TST reaction, including the recipient’s age, simultaneous administration of a live vaccine, concomitant infections, metabolic deficiencies, underlying disease, and improper placement or storage of the PPD solution. Live vaccinations such as the measles, mumps, rubella (MMR) and varicella vaccines may cause a false-negative response to the TST for up to 2 months after immunization. However, results of the TST performed simultaneously with inoculation of these vaccines are unaffected.21 Other potential causes of false-negative test results are listed in Box 235-3. Because there are many potential causes of a false-negative TST reaction, the absence of a positive reaction does not exclude TB disease or infection. Anergy, which is a decreased or absent delayed-type hypersensitivity response, can be caused by severe or febrile illness, miliary or pulmonary disease, and most of the factors listed in Box 235-3. Of all patients with TB, 10% to 25% have negative reactions to the TST. Approximately one third of patients with HIV infection and more than 60% of patients with AIDS have skin test reactions of less than 5 mm, even though they have been infected with M. tuberculosis.7 A negative TST result does not exclude LTBI in patients who are immunocompromised. The usefulness of anergy testing for immunocompromised individuals has not been consistently demonstrated and is no longer recommended.7 False-negative reactions to the TST can also result from a decreased or waning delayed-type hypersensitivity reaction over time, especially among older adults who may have been infected years before being screened for TB. Although they were previously infected with TB, their hypersensitivity to the PPD antigen has been blunted over time. Although they may not respond to the initial skin test, the skin test may stimulate or “boost” their ability to react to the tuberculin on a subsequent test. Therefore, skin testing is repeated in 1 to 3 weeks. A positive reaction to the second test probably represents a boosted reaction rather than a reaction to new infection. On the basis of this two-step testing, the patient should be classified as previously infected, and management should proceed accordingly. Guidelines to interpret the results of a two-step TST are included in Box 235-4.
Tuberculosis
Definition and Epidemiology
![]() Specialist referral is recommended for any patient suspected of having pulmonary or extrapulmonary TB.
Specialist referral is recommended for any patient suspected of having pulmonary or extrapulmonary TB.
Pathophysiology
Clinical Presentation
Physical Examination
Diagnostics
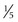 to
to 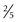 inch) in diameter and disappears within several hours. If a wheal is not produced, the injection was probably too deep and will likely result in a false-negative reading. In the absence of a wheal, the skin test should be repeated. The amount of induration, rather than the erythema, is measured. All reactions, even those classified as negative, should be recorded in millimeters of induration. If no induration is found, 0 mm should be recorded.
inch) in diameter and disappears within several hours. If a wheal is not produced, the injection was probably too deep and will likely result in a false-negative reading. In the absence of a wheal, the skin test should be repeated. The amount of induration, rather than the erythema, is measured. All reactions, even those classified as negative, should be recorded in millimeters of induration. If no induration is found, 0 mm should be recorded.
Tuberculosis
Chapter 235







