INTRODUCTION AND EPIDEMIOLOGY
Tuberculosis remains an important worldwide infection, with more than one third of the overall population harboring the bacterium. It is the second leading cause of death among infectious diseases and a major cause of death among those with human immunodeficiency disease (HIV), especially in countries with limited resources.1,2 Despite therapeutic progress over the past 20 years, drug resistance and HIV coinfection continue to challenge the global control of tuberculosis.2
Tuberculosis has been on the decline in the United States, with an average 3.8% decrease each year from 2000 to 2010.3 This reduction is primarily due to tuberculosis control programs targeting high-risk individuals. In addition, improved infection control policies, increased vigilance among physicians, implementation of directly observed therapy, and standardized drug regimens all contributed to the decline of tuberculosis rates. Although overall national cases have decreased, the incidence in foreign-born patients remains 12 times that of U.S.-born persons.3 In foreign-born patients, clinical tuberculosis is usually from reactivation of latent disease. Overall, reactivation of latent tuberculosis is responsible for 70% of active tuberculosis cases.4
Continued improvement in tuberculosis control and prevention requires recognition and treatment of high-risk populations (Table 67-1). Screening and treatment of latent infection in high-risk individuals are key to reducing tuberculosis in the United States.4
Immigrants from high-prevalence countries Patients with the human immunodeficiency virus Residents and staff of prisons or shelters for the homeless Alcoholics and illicit drug users Elderly and nursing home patients |
PATHOPHYSIOLOGY
Mycobacterium tuberculosis is a slow-growing aerobic rod that has a multilayered cell wall containing lipids that account for its acid-fast staining property. Because the organism is an obligate aerobe, it settles in areas of high oxygen content and blood flow. Transmission from person to person occurs through inhalation of droplet nuclei into the lungs. Persons with active tuberculosis who excrete mycobacteria in saliva or sputum are the most infectious.5 Only 30% of patients actually become infected after a droplet exposure.6
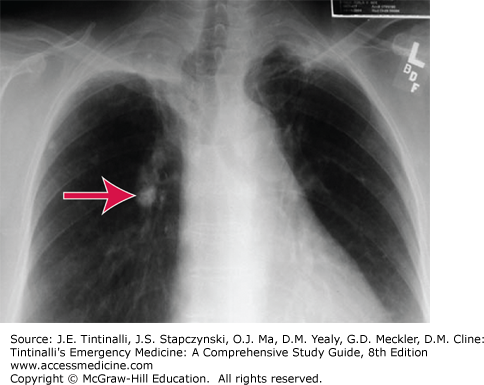
Once the organisms reach the lungs, host defenses are activated. Some organisms survive and are transported to the regional lymph nodes, where the host cell-mediated immunity is activated to contain the infection. Granulomas, known as tubercles, form as a result of this process, which involves activated macrophages and T lymphocytes in addition to active bacteria in most cases. Tubercles are a sign of primary infection and may progress to caseation necrosis and calcification. In the lung, the Ghon complex (Figure 67-1) is a manifestation, appearing as calcified hilar lymph nodes.
If the tubercle fails to contain the infection, the mycobacteria may spread by hematogenous, lymphatic, or direct mechanical routes. The tendency is for survival in areas of high oxygen content or blood flow, such as the apical and posterior segments of the upper lobe and the superior segment of the lower lobe of the lung, the renal cortex, the meninges, the epiphyses of long bones, and the vertebrae. In these areas, the mycobacteria can remain dormant for years. During dormancy (or latent infection), the disease is detected by a positive tuberculin skin test. The skin test generally becomes positive 1 to 2 months after initial exposure. Only 1% to 13% of otherwise healthy patients will go on to develop active postprimary disease. However, children and HIV patients have a higher risk, approaching a 20% frequency of postprimary infection.5,7
Whether latent infection progresses to recurrently active (or “reactivation”) tuberculosis is dependent on the immune status of the host.5,6 As the host defense system weakens, it is no longer capable of containing the foci of previous hematogenous spread, and active tuberculosis may develop. The risk for reactivation is higher among HIV-infected persons and those more than 50 years old.6 In 5% of persons, latent infection may progress to active disease within 2 years after initial exposure, with another 5% developing disease later in life.5,7 In immunocompromised hosts, spread often occurs rapidly, and progression of early active disease is more frequent. HIV-infected patients are at particularly high risk, with progression reported at 7% to 10% per year.5 Other groups at risk for developing tuberculosis activation include those immunocompromised from carcinoma of solid organs, leukemia, transplantation, or medications such as antagonists of tumor necrosis factor-α (etanercept or infliximab) or corticosteroids. Those with select chronic diseases such as diabetes, chronic renal failure requiring hemodialysis, psoriasis, and silicosis are also at increased risk for tuberculosis activation.5,8
CLINICAL FEATURES
The initial infection is usually asymptomatic, often detected only by a positive screening tuberculin skin test or by abnormalities on chest radiograph. When the infection is primary and active, common symptoms include fever, malaise, weight loss, and chest pain.5 Infrequently, a pneumonitis that is similar to a viral or bacterial infection appears. Hilar adenopathy is present but rarely massive. In some cases, especially in immunocompromised patients, the primary infection may be rapidly progressive and fatal.
When latent infection progresses to tuberculosis reactivation, symptoms may be systemic or pulmonary. The most common reactivation symptoms are similar to those of primary tuberculosis and include fever, night sweats, malaise, fatigue, and weight loss. Productive cough, hemoptysis, dyspnea, and pleuritic chest pain develop as the infection spreads within the lungs. Physical examination is generally unremarkable, although rales may be noted in areas of pulmonary infection.
Although most cases of active tuberculosis involve the lungs, up to 20% of cases will have extrapulmonary manifestations.5 The most common extrapulmonary site of tuberculosis is the lymphatic system—painless lymphadenopathy (i.e., scrofula, cervical lymphadenitis). Other extrapulmonary manifestations include abdominal pain due to hepatosplenomegaly, peritoneal tubercles, prostatitis, epididymitis, or orchitis; adrenal insufficiency; bone pain with arthritis, osteomyelitis, or Pott’s disease (bony destruction, often in the spine); hematuria and sterile puree; and meningitis. Tuberculosis can also cause pericarditis, which can lead to tamponade and constrictive symptoms. One key extrapulmonary tuberculosis axiom is that it can mimic many other common diseases, especially in the elderly and HIV patients.
DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS
With the aging population, consider tuberculosis in all patients over 50 years old with a pneumonia-like presentation or prominent respiratory complaint.5,6 Similarly, consider the disease in those with HIV or on immunosuppressive medications (notably after transplantation or with a connective tissue disease). The variable clinical presentation and the time required to culture the organism make diagnosis in the ED challenging. The goal is to have considered and begun testing for tuberculosis and to start respiratory precautions while awaiting results.
The ED is the point of entry into the healthcare system for many patients.9 Prehospital and ED personnel should think of potential tuberculosis in higher-risk patients with lung symptoms, institute appropriate respiratory precautions, and notify healthcare providers about the possibility of tuberculosis. Place patients with suspected tuberculosis in separate waiting areas, provide them with surgical masks, and instruct them to cover the mouth and nose when coughing. Immunocompromised patients with respiratory symptoms should be evaluated promptly and isolated until tuberculosis can be excluded based on a chest radiograph.10 A negative-pressure room is ideal for isolation when available.
During triage or initial assessment, consider the diagnosis of tuberculosis in any patient with a persistent cough (weeks or months) that has not improved despite appropriate treatment. Tuberculosis can mimic community-acquired pneumonia; clues that suggest tuberculosis include hemoptysis, night sweats, and weight loss. On chest radiograph, look carefully for upper lung field involvement, fibrocalcific changes, pleural capping, or a calcified Ghon complex.5
Once tuberculosis is suspected, give empiric antibiotics for pneumonia, admit the patient to an isolation bed, and institute airborne precautions. The evaluation should include sputum culture and tuberculosis skin testing and should also include HIV testing if the patient’s HIV status is unknown.
The most common method for screening for exposure to M. tuberculosis is a skin test. The Mantoux test uses intracutaneous injection of 0.1 mL of purified protein derivative into the forearm. The test relies on a delayed-type hypersensitivity reaction that is triggered in those with past infection or those with a significant recent exposure to tuberculosis. The test is read between 48 and 72 hours after administration by measuring the extent of skin induration at the test site; erythema or other skin changes are not assessed (Table 67-2).10 All persons with a new positive skin test or recent conversion should be referred for treatment of latent tuberculosis.
≥5-mm induration is positive in: Patients with the human immunodeficiency virus Patients with close contact with a tuberculosis-infected individual Patients with abnormal chest radiograph suggestive of healed tuberculosis Patients with organ transplants and other immunosuppressed patients receiving the equivalent of prednisone >15 milligrams per day for >1 month |
≥10-mm induration is positive in patients not meeting the above criteria but who have other risks: Injection drug users High-prevalence groups (immigrants, long-term care facility residents, persons in local high-risk areas) Patients with conditions that increase the risk of progression to active disease (silicosis, diabetes, carcinoma of the head, neck, or lung) Children <4 y of age |
| ≥15-mm induration is positive in all others |
Detection of newly infected persons in a screening program: ≥10-mm induration increase within any 2-y period is positive if <35 y ≥15-mm induration increase within any 2-y period is positive if >35 y If the patient is anergic, other epidemiologic factors must be considered |
In a few situations, a positive skin test is not diagnostic of tuberculosis. Those who received Bacillus Calmette-Guérin (BCG) immunization for tuberculosis prevention will often have a positive skin test response in absence of infection. Exposure to nontuberculosis mycobacteria also can result in a false-positive test. False-negative skin test results occur with improper administration or reading, very early in the disease, or with profound underlying immunosuppression (notably in HIV).7,8,11
Interferon-gamma release assays (IGRA) are blood tests that indirectly assess for tuberculosis. The test seeks the response to peptides present in all M. tuberculosis proteins, which trigger the release of interferon-gamma by the infected host.11 These proteins are absent in the BCG vaccine and in most nontuberculous mycobacteria, making IGRA more specific than skin testing.8,11 IGRA is used in conjunction with a history, chest radiograph, and culture in those with suspected active tuberculosis.11,12 Currently used IGRA tests give results in 16 to 24 hours. These tests are especially helpful when follow-up care compliance is a concern, notably in the homeless or drug-abusing patient, and can aid in those patients in whom skin testing is not helpful for the previously mentioned reasons or with known previous exposure (e.g., a healthcare worker).8,11
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree