Fig. 16.1
First principles: Trauma care can be thought of as a coordinated effort to minimize the duration of impaired oxygen delivery and its far-reaching consequences. The major options for forward transfer (advanced resuscitation, imaging, operative or angiographic control of hemorrhage, ICU care) are all dedicated to the prompt reversal of shock. DO2 = oxygen delivery, Hb = hemoglobin concentration, SaO2 = oxygen saturation, PaO2 = arterial partial pressure of oxygen, CO = cardiac output, PTX = pneumothorax
Since rapid restoration of oxygen delivery is a first principle of trauma care, it follows that all aspects of the structure and process of trauma systems should flow from it and be measured against it. EMS systems are geared toward the expeditious transfer of patients to appropriate facilities for definitive care and toward the efficient handover of patients to responding trauma teams. Effective trauma teams, in turn, coordinate the hospital-based trauma resuscitation, bringing all available resources and personnel (ED, diagnostic imaging, lab, blood bank, operating rooms, respiratory therapy, anesthesia, surgery, etc.) efficiently to the front lines and in the right sequence, to reverse the physiologic consequences of shock. They must maneuver a highly sophisticated and diverse response in conditions of uncertainty, where the depth and nature of shock are often unclear and where, amid the distractions of the resuscitation, more information is constantly arriving. The trauma team leader is at the eye of this storm, analyzing and reanalyzing information, determining a course of action, uniting the team around a common purpose, and paving the way forward.
Early Data Acquisition: The Primary Survey as a Decision-Making Tool
The handover from EMS is the first key transition point in the trauma resuscitation and can provide critical data on a trauma patient’s ultimate disposition. A clear and concise handover is also the launch point for the primary survey and may direct the trauma team to issues requiring special focus and attention to detail.
The primary survey and its adjuncts are designed to provide high priority and immediately actionable clinical data, particularly regarding the presence or absence of shock and its likely causes [6, 7]. Mindful patient assessment and clear reporting of findings are at the core of the trauma team response. A single clinician should be designated to complete the primary survey and to report findings in a clear and concise manner. This communication is a key source of raw data that will guide the team in the major decisions that lie ahead. In some cases, as few as two data points are sufficient to prompt decisive action, for example, the presence of penetrating chest trauma and profound hypotension may trigger a decision to initiate a resuscitative thoracotomy. The primary survey should be concurrently completed and reported within a few minutes and should be repeated until a clear picture of injury pattern has emerged, key assessments have been completed, critical interventions have been performed, a measure of stability has been attained, and onward disposition has been determined. The adjuncts to the primary survey (Table 16.1), once completed, are important sources of information in the resuscitation effort. Their results should be announced and documented as each becomes available and, where possible, should be prominently displayed.
Table 16.1
Adjuncts to the primary survey as shock resuscitation tools
Adjunct | Application in shock resuscitation |
|---|---|
ECG | Blunt cardiac injury, acute versus chronic cardiac disease (e.g., acute coronary syndrome) |
SaO2 | Peripheral perfusion |
BP | Presence of shock |
ABG | Base deficit—presence of shock |
Lactate—presence of shock | |
EtCO2 | Tube placement, adequacy of circulation |
Gastric | – |
Foley | Urine output for severity of shock |
CXR | Evaluate sources of hemorrhagic shock |
PXR | Evaluate source of hemorrhagic shock |
EFAST | Evaluate source of hemorrhagic shock |
T | Adjunct treatment in hemorrhagic shock |
The primary survey and its adjuncts should begin to create a clear picture of a trauma patient’s clinical status and should begin to outline and prioritize available courses of action. Each new data point will further support or refute a trauma team leader’s hypothesis regarding the nature and severity of shock and will refine the decision-making process.
Weighing the Options
While the emergency department (ED) is an appropriate place for the initial assessment and early resuscitation of trauma patients, it is not a place for definitive diagnosis or treatment, and no strong gains in either area will be made as long as patients are left there. In general, time in the ED should be minimized. However, as soon as a patient is moved from the resuscitation area, he or she will encounter new risks that must be weighed against the benefits of this movement.
For hemodynamically unstable patients, the choice of transfer destination is relatively straightforward. Shock patients exhibiting either no response or a fragile transient response to early resuscitative measures should be taken immediately to the operating room (OR). A single hypotensive episode, which reflects over 1500 mL of blood loss, may be enough to determine the presence of hemodynamic instability, although some clinicians will add an assessment of metabolic acidosis (base deficit, lactate) to more definitively confirm the presence of shock before making a commitment to go to the OR [8]. The decision to go to the OR with an unstable patient can occur early on in the course of the resuscitation, often on the basis of only a few data points, and in this case, the remainder of the resuscitative effort is dedicated to getting the patient ready to move, ensuring that key details of the resuscitation are completed (e.g., adjuncts to primary survey, cross match), and preparing the accepting team in the operating room for the next phase of patient care. The transition to the operating room is described in more detail below.
For patients with relative hemodynamic stability, the decision to pursue an operative intervention is not as urgent, and there are more diagnostic and therapeutic options available. It should be remembered however, that many of these patients, particularly those who have been injured recently, may still be in compensated shock, with undetected ongoing bleeding. Any evidence of hemodynamic deterioration should redirect patients to an operative algorithm. Until such deterioration occurs, trauma teams will be faced with the following six options:
1.
Sit tight and wait for a signal: When a patient’s hemodynamic status is unclear, a period of intense watchful waiting in the secure surroundings of the trauma resuscitation area may be warranted. It should be emphasized, that this is a period of active observation, with serial determination of vital signs, repetition of the physical examination, and serial determination of the presence of occult shock through the measurement of base deficit and lactate, with special focus on how these parameters respond to initial resuscitation. The primary survey should be iterative, and the results of the adjuncts should be carefully reviewed to determine potential etiologies for shock. During a brief period of watchful waiting, the appropriate transfer destination may become clear—a patient considered to be a transient responder may need to go to the operating room, while a stable patient may be safely transferred to CT for definitive imaging.
2.
Computed tomography: Taking a trauma patient to CT is a well-known pitfall in trauma care. Trauma teams should always recognize the potential for a patient in compensated, undetected shock to decompensate in the scanner, where access to the patient can be limited and resuscitative equipment and personnel are less available. However, the speed of modern CT scanners and their geographic location, often steps away from the resuscitation area, have reduced the risk of a trip to CT. The advantages of CT, both for blunt trauma as a definitive imaging tool and penetrating trauma, to map trajectory and establish follow-up diagnostic and therapeutic approaches, are great. Still, trauma teams must confirm hemodynamic stability before traveling to CT.
Since CT is a default path for the majority of stable multi-trauma patients and since it is a time-dependent priority in patients with traumatic brain injuries, resuscitative efforts in the primary survey are often directed toward moving the patient efficiently from the trauma bay to CT. The TTL should remain vigilant about and immediately address lulls in the progress of this transfer. If there is any concern about the need for operative intervention after the scan, the OR and relevant surgical services (e.g., neurosurgery) should be informed and on standby so that a time-consuming transfer back the ED can be avoided.
3.
Angiography: The role for angiography in unstable patients is limited to a very few clinical scenarios. Some groups of investigators have advocated for the use of angiography for the control of hemorrhage in patients with solid organ and pelvic injuries whose hemodynamics can be maintained with active resuscitation (transient responders) [9, 10]. However, extreme caution must be exercised when contemplating such a move, and the full spectrum of resuscitative capabilities and personnel should be transferred to the angiography suite along with the patient. Resuscitation can be difficult under these circumstances, with a sterile field and radiography limiting access to the patient and without the usual equipment available for resuscitative procedures. Furthermore, since most hemorrhagic injuries of the liver and pelvis are venous, angiography may be of limited utility in many of these injuries. That said, if no surgically accessible injuries are identified in the primary survey and a patient’s hemodynamic stability can be maintained with a carefully controlled resuscitation, the risks of angiography may be justifiable. In general, angiography usually follows CT, where contrast extravasation in the arterial phase pinpoints specific areas of active arterial hemorrhage, and CT rules out the immediate need for operative intervention [11]. For patients with suspected pelvic fractures and bladder injuries, pelvic angiography should be completed before retrograde urethrograms or cystograms to ensure vascular contrast extravasation in the pelvis can be detected.
4.




Operating room: The operating room is an ideal destination for patients in shock. With dedicated surgeons, anesthesiologists and nurses present, and a full spectrum of resuscitative options and equipment available, resuscitation can be intensified and titrated against continuous hemodynamic monitoring. With better lighting, sterile technique, and equipment, procedures such as chest tube and line placement, exploration of wounds, and completion of resuscitative thoracotomy can be done more safely and more precisely. However, for unstable patients, a partially unmonitored move to an incompletely prepared operating theater can be hazardous. Transfer to the operating theater is a critical transition (Fig. 16.2), whose benefits can be maximized by careful mitigation of risks. Some trauma centers have full operating theater capabilities in the resuscitation area, allowing the resuscitation to blend seamlessly with definitive surgery for damage control. Locating surgical capabilities in the resuscitation area has been associated with better outcomes for some of the most unstable patients [12]. When such capabilities are not available, it may still be possible to create a smooth transition between the trauma bay and the operating room. The OR should be part of the trauma activation callout and OR personnel, including anesthesia and nursing, should begin to arrange operative capacity for a potential imminent transfer. The trauma team leader and the OR should be in close communication throughout the resuscitation, and the decision to transfer to the OR and the anticipated resource and equipment needs should be specified as early as possible (Fig. 16.3). Some operating theaters have prespecified trauma laparotomy trays, complete with vascular and thoracic instruments, along with appropriate retractors, and even sternal saws that can be rapidly opened as needed. It is important that the OR setup for trauma is predefined, but even if it is, it is useful for the TTL to conduct a briefing to ensure that the assembled team is aware of the risks, expected operative strategies, and anticipated equipment needs. Ideally, the trauma patient will arrive from the trauma bay to a fully staffed, warm operating room, with monitoring interfaces and operative instruments set up and ready to go. Even, or perhaps especially, in this operative crisis situation, a preoperative briefing and a time-out, as defined by the WHO Safe Surgery Saves Lives campaign, are essential to set the direction for the case [13].
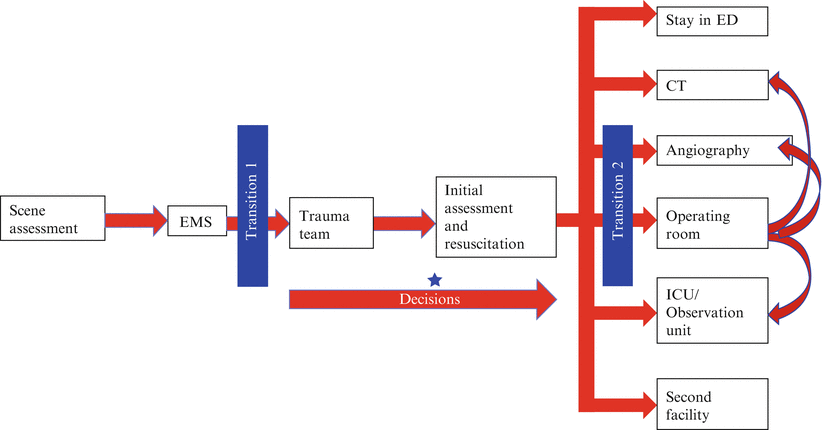


Fig. 16.2
The process of trauma care: key transitions and decision points. Early decisions regarding the need for damage control resuscitation, massive transfusion, and disposition from the trauma bay and (onward) can be made very early in the course of resuscitation

Fig. 16.3
An example of a checklist describing key priorities before transfer from the trauma bay to the operating room
In some cases, a thorough primary survey, including chest and pelvic X-rays and an extended focused assessment with sonography for trauma (eFAST) ultrasound exam of the chest and abdomen, may not reveal a clear, single cause of hemodynamic instability, and an unstable patient may arrive in the operating room without a clear operative plan. Some operating rooms are equipped with diagnostic modalities and expertise, including transesophageal echocardiography, X-ray, ultrasound, interventional radiology, and an upper and lower gastrointestinal endoscopy to quickly repeat or intensify the workup of undifferentiated trauma patients. When the source of hemorrhage is difficult to identify (when diagnostic imaging is not directive), the abdomen is the usual suspect. Investigators at the University of Southern California (USC) observed that exploratory laparotomy was required much more frequently than exploratory thoracotomy (89 % versus 1.9 %) for unstable patients with thoracoabdominal trauma. They suggested abdominal exploration should be prioritized over thoracic exploration in this instance [14]. The team should, however, be prepared to do both, although this was rarely required in the USC series (2.1 %). Operative interventions in hemodynamically unstable patients should have defined objectives and clear onward disposition options. Hemodynamic instability is often associated with acidosis, coagulopathy, and hypothermia, which justify a damage control surgical approach that prioritizes early control of hemorrhage and contamination and abbreviation of operative efforts [15]. TTLs can decide on this course of action even before a patient’s arrival in the operating room and should know the onward disposition from the operating theater even before a patient arrives there. For example, if a traumatic brain injury was suspected, but a CT of the head could not be obtained in advance of an urgent exploratory laparotomy, arrangements can be made for immediate postoperative transfer to CT. Alternatively, if a patient with a complex pelvic fracture is taken to the operating room for thoracic or abdominal injuries, the interventional radiologists and angiography suite can be readied in advance for possible direct transfer from the operating room to the angiography suite. Finally, if operative intervention has been successful in controlling hemorrhage, aggressive restoration of homeostasis is best accomplished by rapid, seamless transfer to a briefed and prepared intensive care unit (ICU).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






