(1)
Division of Pulmonary and Critical Care Medicine, Eastern Virginia Medical School, Norfolk, VA, USA
Keywords
ToxicologyOverdoseSubstance abuseCocaineEthylene glycolAcetaminophenNaloxoneFlumazenilGastric lavageActivated charcoalToxidromesSalicylatesEthanolMethanolThis chapter provides a brief overview on the management of patients following an accidental or suicidal overdose. The reader is referred to toxicology texts and their local poison center for information on the management of specific intoxications.
General Measures
Stabilization of patient, i.e. airway, breathing and circulation
Intubate comatose and seizing patients
Obtain IV access
Treat hypotension initially with volume expansion
Comatose patients should be given naloxone 0.8 mg IV
Specific antidotes are available for a limited number of intoxications (see Table 45.1)
Table 45.1
Toxic substances with specific antidotes
Agent
Antidote
Acetaminophen
N-acetylcysteine
Anticholinergic poisoning
Physostigmine
Anticoagulants
Vitamin K, protamine
Benzodiazepines
Flumazenil
Beta-adrenergic antagonists
Glucagon, calcium salts, isoproterenol
Carbon monoxide
Oxygen, hyperbaric oxygen
Cholinergic syndromes
Atropine
Digoxin
Fab antibody, Mg
Ethylene glycol
Fomepizole, thiamine, ethanol
Fluoride
Calcium and Mg salts
Heavy metals
BAL, DMSA, d-penicillamine
Iron
Desferoxime
Isoniazid
GABA antagonists, pyridoxime
Methemoglobinemia
Methylene blue
Opioids
Naloxone
Flumazenil (a benzodiazepine antagonist), may be indicated in patients who present with obtundation or coma following the ingestion of benzodiazepines. Flumazenil is contraindicated in patients with mixed overdoses (tricyclic antidepressants and benzodiazepines) as well as patients with a history of seizures. An initial dose of 0.2 mg intravenously should be given over 30 s. Additional doses of 0.2–0.5 mg can be given up to a total of 3 mg
Ipecac is not recommended for ingestions treated in hospital. May be used at home for accidental ingestions in children. Should not be given after ingestion of caustic substances and acids
Gastric lavage is indicated in the following circumstances:
recent ingestion (<1 h) of a potentially life-threatening poison
ingestion of a substance that slows gastric emptying (e.g. anticholinergic medications)
ingestion of a poison that is slowly absorbed from the gastrointestinal tract
ingestion of a substance that does not bind well to activated charcoal (see below)
ingestion of specific life-threatening poisons (e.g. tricyclic antidepressants, theophylline, cyanide)
Contraindicated in caustic ingestions
Technique for Performing Gastric Lavage
Patients who cannot protect their airway MUST BE INTUBATED prior to performing gastric lavage
Patient placed in head down lateral position
Place large bore lavage tube through mouth
Aspirate to empty stomach
Lavage with 150–300 mL tepid tap water
Activated Charcoal
This is the cornerstone of the management of most ingestions. Activated charcoal is administered in a dose of 50–100 g.
Drugs not well bound to activated charcoal
bromides
caustics
cyanide
ethylene glycol
heavy metals
iron
isopropyl alcohol
lithium
methanol
Drugs amenable to repeat-dose activate charcoal therapy
carbamazepine
diazepam
digitalis
phenobarbital
phenytoin
salicylates
theophylline
tricyclic antidepressants
Hemodialysis/Hemoperfusion
Some drugs are cleared by hemodialysis/hemoperfusion; this technique should be instituted as clinical circumstances dictate.
Hemoperfusion
acetaminophen
theophylline
methotrexate
phenylbutazone
procainamide
quinidine
Hemodialysis
ammonium chloride
amphetamine
atenolol
meprobamate
methyldopa
nadolol
phenobarbital
procainamide
quinidine
sotalol
thallium
ethanol
methanol
ethylene glycol
isopropanol
aspirin
lithium
bromide
arsenic
dabigatran
In the evaluation of the patients with a possible drug overdose it is useful to look for symptom complexes or “toxidromes” that may help in identifying the type of drug ingested. The following toxidromes should be identified:
Depressed level of consciousness
coma, stupor, lethargy, confusion
Anticholinergic signs
mydriasis, increased blood pressure, tachycardia, warm dry skin, erythema, delirium, hallucination, urinary retention
Cholinergic signs
salivation, lacrimation, urination, defecation (SLUD), miosis, bradycardia, sweating
Sympathetic signs
high blood pressure, tachycardia, hyperthermia, mydriasis
Serotonin syndrome
Confusion, myoclonus, hyperreflexia, diaphoresis, tremor, flushing, diarrhoea, fever
Neurological signs
Nystagmus, tremors, hyperreflexia, seizures, extrapyramidal signs, hallucinations
Common Agents Responsible for
Depressed level of consciousness
alcohols
anticholinergic
anticonvulsants
antidepressants
antihistamines
antipsychotics
barbiturates
benzodiazepines
carbon monoxide
opiates
sulfonylureas
Seizures
phenytoin
beta-blockers
clonidine
theophylline
meperidine
amphetamines
cocaine
Anticholinergic syndrome
antidepressants
antihistamines
antipsychotic
belladonna alkaloids
mushrooms
Cholinergic syndrome
insecticides
mushrooms
nicotine
Sympathetic syndrome
cocaine
amphetamines
phenycyclidine
Serotonin syndrome
SSRI: fluoxetine etc
isoniazid
meperidine
clomipramine
Extrapyramidal
antipsychotic
Nystagmus
alcohols
lithium
carbamazepine
Hallucinations
amphetamines
cocaine
phencyclidine
cannabinoids
Common Intoxications
Acetaminophen
Acetaminophen (acetyl-para-aminophenol or APAP) is an active ingredient of several hundred preparations and is the most common drug implicated in both accidental (children) and suicidal overdoses. In 2003, the American Association of Poison Control Centers reported more than 127,000 exposures involving acetaminophen [1]. Of these exposures, 65,000 patients received treatment in a medical facility, and 16,500 received N-acetylcysteine (NAC). There were 214 deaths involving overdose where an analgesic agent was thought to be primarily responsible. In 62 of these cases, APAP was the single agent involved. APAP toxicity is the major cause of fulminant hepatic failure (FHF) and is implicated in as many as 39 % of cases presenting to tertiary care hospitals.
Acetaminophen is well absorbed, with peak levels about 4 h after an overdose. After therapeutic doses approximately 90 % of acetaminophen is conjugated by the liver to nontoxic inactive compounds which are renally excreted. About 5 % is excreted unchanged in the urine and about 5 % is oxidized by the P-450 mixed function oxidase enzyme to yield highly reactive toxic intermediates which are detoxified by reduced glutathione. After overdosage, the amount of drug metabolized by the P-450 route is increased. The same process occurs in the kidney, and while renal toxicity may occur with acetaminophen overdose it is far less common than hepatotoxicity. The hepatotoxicity may vary from asymptomatic elevation of liver enzymes to fatal liver failure. Pancreatitis and myocardial necrosis has also been described. It should be noted that stores of reduced glutathione are diminished in alcoholics and malnourished patients, predisposing to hepatic toxicity at therapeutic dosages. Ingestions of greater than 7.5 g in an adult should be considered potentially toxic.
Non-acute ingestions of APAP, frequently referred to as subacute or chronic, are ingestions that take place over a period longer than 4 h. In these cases, the nomogram (Fig. 45.1) offers no guidance in treatment, because it is intended only for use with acute ingestions. Most cases of nonacute ingestion of APAP that result in hepatotoxicity involve persons taking supra-therapeutic doses who are at increased risk for APAP-induced hepatotoxicity. However, hepatotoxicity has been reported in patients taking “therapeutic doses” (4 g/day); these patients usually have other risk factors including alcohol abuse, malnutrition, underlying liver disease or concomitant drugs (including phenytoin) [2, 3].
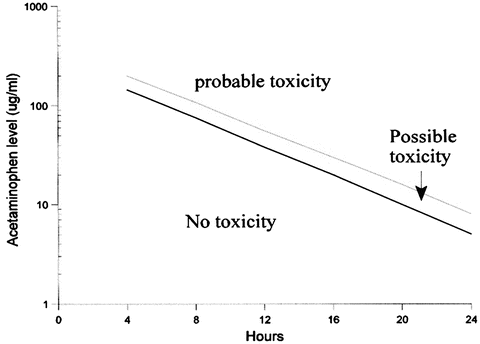

Fig. 45.1
Acetaminophen treatment nomogram
Watkins et al randomized 147 healthy volunteers to receive, acetaminophen (4 g day), an oral opiate, acetaminophen + an opiate, or placebo [4]. In this study, the daily intake of acetaminophen was associated with ALT elevation (>3 times) normal in up to 44 % of patients (risk not influenced by concomitant opiate). The AST levels began to rise on the 5th day and peaked on the tenth day when the drug was stopped. A prospective study of more than 600 patients from 22 US tertiary care centers found that acetaminophen related liver damage is the leading cause of acute liver failure in the country and that about half of such cases involved unintentional overdose [5]. In the unintentional group, 38 % took two or more acetaminophen preparations simultaneously. Based on the risks of hepatotoxicity with the “current dosage” recommendations the FDA has required a change in the labeling of these products, including reducing the maximum daily dose for both prescription and over-the counter acetaminophen products from 4 g per day to 3,250 mg per day (500 q 4 hourly or 650 q 6 hourly), limiting the dose in individual tablets, and adding to the labeling of these products a warning that individuals who chronically use alcohol should use an even lower dose of the drug [6, 7].
Signs and Symptoms
Stage 1: 12–24 h after ingestion; asymptomatic or mild GI symptoms
Stage 2: 24–72 h; right upper quadrant pain, nausea and vomiting, liver enzymes begin to rise.
Stage 3: 72–96 h; maximal hepatic injury
Stage 4: 4 days to 2 weeks; patient either improves with normalization of enzymes or progresses to acute hepatic necrosis with liver failure
Management
Analyzing the serum acetaminophen concentration is essential in all cases of acute overdose (see Fig. 45.1). A level prior to 4 h is difficult to interpret. High potential for toxicity exists when serum concentration is >200 μg/mL at 4 h, 50 μg/mL at 12 h and 7 μg/mL at 24 h after ingestion.
N-acetylcysteine (NAC) should be administered as soon as possible within the first 24 h of ingestion. However, antidotal therapy is optimal when given within 12 h of acetaminophen ingestion. NAC should be given if the patient has ingested more than 140 mg/kg (or 10 g) acetaminophen, if the serum level is above 140 μg/mL, or if the serum level is in the toxic range. The dose of N-acetylcysteine is 140 mg/kg as an initial oral loading dose, followed by 70 mg/kg every 4 h for a total of 17 doses. Nausea and vomiting are reported in 33 % of APAP overdoses before NAC and in an estimated 51 % during oral NAC therapy. In 2004, Acetadote became the first NAC solution approved by the FDA for IV use, allowing the United States to join the rest of the industrialized world, which has been using IV NAC since its introduction in 1977 [8]. A 72-h oral, 48-h IV, and 20-h IV protocol have been reported. These protocols have been compared by retrospective study and through meta-analysis [9]. When started within 8 h of ingestion, no protocol shows advantage over another. However, the 20-h IV protocol is generally preferred; this is given as a loading dose of 150 mg/kg over 15 min, followed by 50 mg/kg infused over 4 h and 100 mg/kg administered over 16 h as a constant infusion. Although there is general consensus that IV administration is preferable in the face of intractable vomiting, no study shows clear evidence that IV therapy is more or less effective than oral NAC therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





