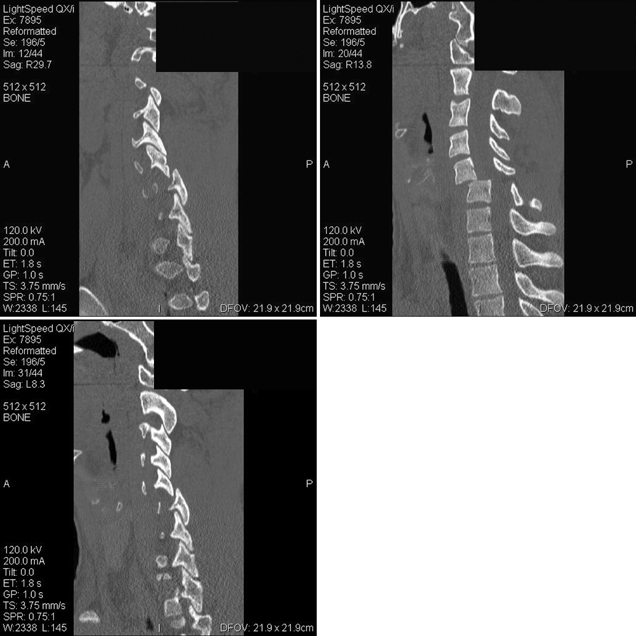
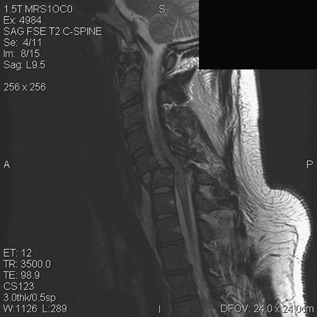
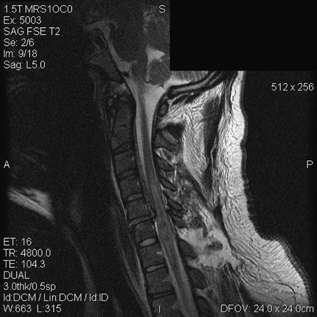
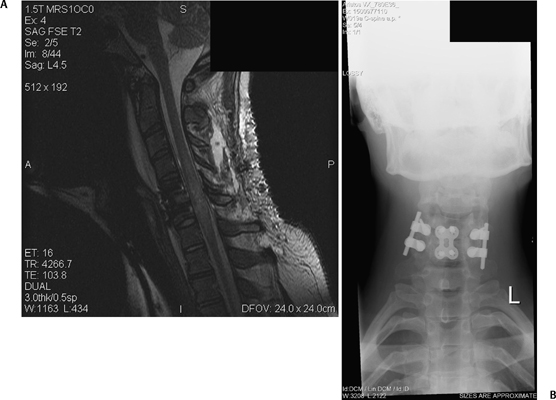
8 Timing of Surgery for Spinal Injuries Gordon K. T. Chu and Michael G. Fehlings A 24-year-old man was admitted to our institution following a blunt assault. On admission, the patient had complete injury at the C6 level. He had an admission American Spinal Injury Association (ASIA) grade of A, and he sustained no other injuries. His admission computed tomography (CT) and magnetic resonance imaging (MRI) are shown in Figs. 8.1 and 8.2. The MRI showed no evidence of disk herniation. He was then taken to the operating room for a closed reduction under fluoroscopic guidance followed by posterior fixation. This was achieved between 12 and 24 hours after arrival. A repeat MRI revealed some continued cord compression secondary to a disrupted disk (Fig. 8.3), and the patient underwent an anterior diskectomy and fusion the following day. Fig. 8.1 Computed tomography (CT) sagittal reconstruction of the C5-C6 flexion distraction injury described in the case study. There is a 60% maximum canal compromise as measured by both CT and magnetic resonance imaging (MRI).1,2 Fig. 8.2 T2-weighted MRI with sagittal image. There is increased signal in the spinal cord at the C5-C6 level, indicative of possible hematoma formation. There is no disk herniation noted. Fig. 8.3 T2-weighted MRI after reduction and posterior fixation. There is some continued cord compression from a disrupted disk, which necessitated an anterior diskectomy. Although a major effort was made to intervene urgently, the extent of the trauma resulted in some continued cord compression that was not completely resolved until at least 48 hours after injury (Fig. 8.4). Twelve months later, his ASIA grade had improved to B. This case illustrates one of the most important unresolved questions in the operative management of spinal cord injuries (SCIs), namely, the timing of surgery to decompress the spinal cord. Fig. 8.4 (A) T2-weighted MRI midsagittal image and (B) the final construct of the patient after decompression and stabilization approximately 1 week after the injury. There is a high-intensity signal at the C6 level, indicative of ongoing edema and early cystic changes. Table 8.1 Spinal Cord Injury Characteristics
| Treatment | Currently no adequate treatment to restore function |
| Cases | > 12,000 per year in North America |
| Affected individuals | 300,000 live with SCI (many being young or middle-aged) |
| Costs | • > $7 billion in 1995 |
| • Increased life span = increased cost of care |
Would earlier intervention in this case (i.e., less than 24 hours after injury or sooner) have resulted in increased recovery? Despite advancements in imaging, critical care, operative technique, and instrumentation, it is still unknown if there is an advantage to early decompression of the spinal cord and what the time window is after injury for decompression to have the maximal benefit. This chapter discusses the controversy surrounding this topic and examines the latest data on the subject.
Spinal cord injury has been acknowledged as a severe injury since ancient Egyptian times, yet there is still no adequate treatment to restore function. Every year in North America, there are over 12,000 cases of SCI. Currently, close to 300,000 people live with SCI, of which many are still young or middle-aged.3–5 The costs directly associated with SCI was in excess of $7 billion in 1995. With improving medical and surgical techniques, the life span of SCI patients will lengthen; therefore, the costs related to their ongoing care can only increase (Table 8.1).6 It is imperative that we in the medical community find a way to change the limited prognosis of these patients.
There is currently no definitive standard for the surgical and medical management of SCI. This is due to an incomplete understanding of the pathophysiology underlying SCI5,7,8 and a scarcity of class I data (Table 8.2) with regard to treatment. Careful scientific studies of SCI have categorized the injury as primary or secondary (Table 8.3). The primary injury mechanism consists of a physical deformation and destruction of spinal cord tissue at the time of injury. It is not amenable to treatment. The primary injury triggers a secondary cascade of events, resulting in continued destruction of the spinal cord; this cascade is known as the secondary injury. Secondary injury resulting in further deterioration of the spinal cord is due to (1) vascular abnormalities, (2) biochemical and electrolyte shifts, and (3) cellular and molecular changes. There is ischemia, loss of autoregulation, and hemorrhage after SCI, as well as calcium and sodium influx and increased extracellular concentration of potassium and excitatory neurotransmitters, such as glutamate, serotonin, and catecholamines. Cellular and molecular changes include infiltration of inflammatory cells, loss of mitochondrial potential, and activation of caspase proteins leading to forms of programmed cell death, including apoptosis. These secondary events can occur from seconds to days to possibly weeks after the primary injury and therefore would afford physicians a chance to intervene and perhaps improve recovery.
| Class of Evidence | Type of Study |
|---|---|
| I | Randomized controlled clinical trial |
| II | Prospective, nonrandomized study |
| III | Retrospective study, case series, expert opinion |
Table 8.3 Spinal Cord Injury Categorized
| Primary (immediate) | • Defined: physical deformation and destruction of cord tissue at the time of injury |
| • Not amenable to treatment | |
| • Triggers cascade of events resulting in progressive destruction of cord (secondary injury) | |
| Secondary (days to weeks) | Further deterioration of spinal cord, due to |
| 1. Vascular abnormalities: ischemia, loss of autoregulation, and hemorrhage | |
| 2. Biochemical and electrolyte shifts: calcium and sodium influx, increased extracellular concentration of potassium, and increased concentration of excitatory neurotransmitters, such as glutamate, serotonin, and catecholamines | |
| 3. Cellular and molecular changes: infiltration of inflammatory cells, loss of mitochondrial potential, caspase activation resulting in apoptosis |
The National Acute Spinal Cord Injury Study (NASCIS) II and III showed that methylprednisolone therapy may improve the neurologic outcome modestly if started within 8 hours of injury (Table 8.4).9,10 NASCIS III suggests that treatment started within 3 hours of injury need only be for 24 hours, as opposed to 48 hours when started later than 3 hours and up to 8 hours after injury. Treatment with methylprednisolone is aimed at arresting several of the above-mentioned mechanisms. This treatment remains controversial due to the analysis of the data from these randomized trials. One important aspect to these studies is that they are the very first randomized controlled trials to imply a potential therapeutic time window for SCI. This partly validates the secondary injury hypothesis. However, this concept of a therapeutic time window not only is attractive for medical therapies but may also apply to surgical treatment.11–15
Table 8.4 Methylprednisolone Therapy in Improving Neurologic Outcomes
| NASCIS | National Acute Spinal Cord Injury Study |
| NASCIS II and III9,10 | • Showed methylprednisolone therapy may modestly improve neurologic outcomes if given within 8 hours of injury |
| • If given within 3 hours of injury, the duration of treatment required is 24 hours | |
| • If given after 3 hours but within 8 hours of injury, the duration of treatment required is 48 hours | |
| Treatment effects | • Halt several of the secondary injuries seen in SCI |
| • Remain controversial |
 The Role of Nonsurgical Management Alone in Spinal Cord Injury (Table 8.5)
The Role of Nonsurgical Management Alone in Spinal Cord Injury (Table 8.5)
The uniformly poor outcomes of SCI have led physicians and surgeons universally to have a fatalistic approach to the treatment of these patients. Patients with SCI secondary to burst fractures and dislocations were routinely untreated surgically in the past. This belief was strengthened by studies demonstrating poorer outcomes in patients whose spinal cords were decompressed with laminectomies and higher complication rates, including neurologic injury following surgery.16–19 Therefore, to justify surgical intervention at all in these patients, the outcomes of nonoperative treatment must be carefully assessed. Class I evidence (Table 8.2) comparing outcomes of operative versus nonoperative patients is lacking. There is only class III evidence from retrospective studies in which to analyze outcomes. Frankel et al,20 in their study of 612 conservatively managed patients, found that 29% of the Frankel A grade patients improved at least one neurologic grade. Instability was noted in only four patients. Others have also shown neurologic improvement with conservative management. In contrast, Katoh et al21 demonstrated that 10% of patients with incomplete cervical cord injury deteriorated with nonsurgical treatment. A prospective nonrandomized case-controlled study of 208 patients by Tator et al22 showed that surgery resulted in a lower mortality than conservative management despite an increased incidence of thromboembolic events. However, no difference in neurologic recovery between the groups could be determined, and the issue of timing of surgery was not examined. Given the absence of class I evidence, nonoperative management cannot be excluded as a valid treatment option, especially for medically unstable patients. However, it is the opinion of most spinal surgeons that advances in neuroanesthesia, critical care, instrumentation, and surgical technique have allowed spinal stabilizations and not just laminectomies to be performed safely with minimal morbidity when compared with how they were performed in the past. These stabilization procedures enable earlier mobilization and easier respiratory care for the patient. Nonoperative management may still be warranted for patients with central cord syndrome; however, nonoperative treatment of SCI is no longer the preferred treatment option.23
 Experimental Studies of Early Decompression (Table 8.6)
Experimental Studies of Early Decompression (Table 8.6)
Early surgery following SCI has been shown to be of benefit in animal models.24–27 Dimar et al,27 using a rat model of SCI whereby the cord was injured with a weight drop and subsequent compression maintained by epidural spacers, demonstrated that minimizing the time of compression results in improved recovery. They had compressed the cord for 0, 2, 6, 24, and 72 hours after the weight drop and assessed hind limb recovery over a 6-week period. The functional recovery was better the shorter the time of compression throughout the 6-week period. Using a dog model, Carlson et al26 concluded that greater neurologic improvement occurred with earlier decompression. They used a hydraulic piston to produce spinal cord compression for 30 or 180 minutes. Following the compression, somatosensory evoked potentials and motor tests were conducted for 28 days. The dogs with the shorter period of compression were able to recover the evoked potentials within 1 hour following decompression compared with no recovery in the 180-minute group. The motor recovery was also much improved in the early decompression group. Two weeks after the injury, the 30-minute compression group had significantly better results with balance, cadence, stair climbing, and climbing an inclined plane. Furthermore, histologic evaluation of the spinal cords revealed that increased compression time resulted in increased lesion volumes of the spinal cords.
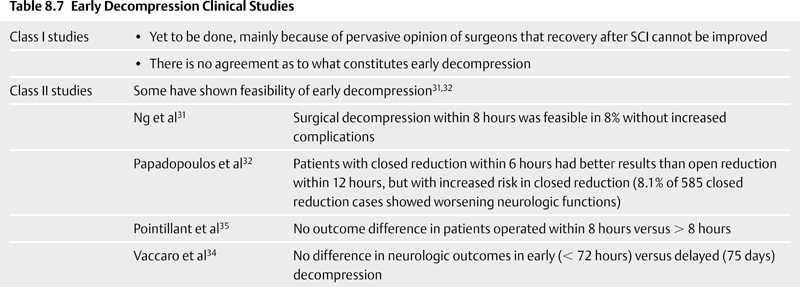
 Clinical Studies of Early Decompression in Spinal Cord Injury (Table 8.7)
Clinical Studies of Early Decompression in Spinal Cord Injury (Table 8.7)
Though animal data have shown the potential for early decompression, human trials have yet to show a definitive answer. There is no class I evidence demonstrating a superior outcome with early decompression. In fact, there have been no class I studies at all with respect to this issue. This is due to a variety of factors; chief among them is the pervasive belief of many surgeons that recovery after SCI cannot be improved. This belief stems from past studies that demonstrated surgery’s worsened outcome (as described earlier in this chapter). Pollard et al,28 in a retrospective review of 412 patients with traumatic, incomplete cervical SCIs with an average follow-up time of 2 years, concluded that the most important prognostic factor for improved recovery was the completeness of the original injury. They also found that younger patients with incomplete injury or either a central cord or Brown-Séquard syndrome had a favorable prognosis. However, they did not find any data to support early surgical intervention or high-dose steroid administration.
Table 8.6 Experimental Decompression Studies
| Dimar et al27 (rat model) | • Minimizing compression time results in improved recovery |
| • Compressed cord for 0, 2, 6, 24, and 72 hours; hind limb recovery was then assessed over a 6-week period | |
| • Results: better functional recovery in group with shorter compression time | |
| Carlson et al26 (dog model) | • Showed greater neurologic improvement with early decompression |
| • Compressed cord for 30 or 180 minutes | |
| • Results: | |
| • Somatosensory and motor tests done showed dogs with shorter decompression were able to recover evoked potentials and motor function within 1 hour, whereas those with 180-minute decompression showed no recovery | |
| • 2 weeks postinjury: 30-minute decompression group showed better balance, cadence, stair climbing, and climbing inclined plane | |
| • Histologically: increased compression time = increased spinal cord lesion volumes |
The controversy surrounding methylprednisolone administration and the general lack of dramatic effect from other medical therapies may also have added to this overall poor attitude regarding recovery from SCI. NASCIS has numerous detractors and the GM1 ganglioside (Sygen) study, despite showing promising initial results, ultimately did not show a significant improvement over methylprednisolone.29,30 There is also no agreement as to what constitutes early decompression in humans. Is the time window 8 hours, as it is with methylprednisolone therapy, or is it 12 hours, or is it earlier? This concept of what constitutes early decompression is controversial. It is uncertain whether any animal studies can be directly translatable, given that clinical SCI is so varied with respect to mechanisms and force when compared with experimental models. Several class II studies have shown the feasibility of early decompression.31,32 Using less than 25 hours as the definition of early surgery and drawing from the NASCIS II database, Duh et al33 concluded that early decompression was beneficial compared with nonsurgical management, although there was no statistical significance. They also noted that surgery after 200 hours (delayed) may also improve outcomes. Another study on 91 patients with cervical SCI demonstrated that a greater percentage of patients with early surgery (< 10 hours) had better neurologic recovery than the control group.32 However, this was a prospective nonrandomized trial, though the authors did demonstrate the achievability of early surgery, as all but one patient underwent surgery within 9 hours of arrival at the hospital. In contrast, Vaccaro et al34 reported in a prospective, randomized clinical trial that early decompression (< 72 hours) resulted in no difference in neurologic outcome compared with delayed surgery (> 5 days). However, there are two main criticisms with this study. First, nearly one third of the patients were lost to follow-up. Second, the authors defined early surgery as < 72 hours, but it has been argued that 72 hours is too long a delay to constitute early decompression. Given these reasons, this study should be considered class II evidence.